Mindray is Elevating Cybersecurity Measures to Tackle Today’s Healthcare Challenges
Mahwah, N.J. 06-04-2024

Mindray BeneVision N-Series Patient Monitoring Systems Achieve UL Solutions Cybersecurity Assurance Program Certification to
UL 2900-2-1
Mindray North America, a global leader and developer of healthcare technologies and solutions for patient monitoring, anesthesia, and ultrasound, announced a new strategic certification that aligns with its secure by design approach to cybersecurity. Its flagship patient monitoring offering, the BeneVision N12, N15, and N17 monitors have obtained the UL Solutions Cybersecurity Assurance Program (CAP) certification to UL 2900-2-1, setting a new standard as the first medical devices manufactured in China to achieve this certification. UL Solutions is a global safety science leader in independent third-party testing, inspection and certification services and related software and advisory offerings.
“Driven by challenges with cybersecurity in healthcare today, Mindray looked to a more comprehensive approach to third-party assessment of the entire product lifecycle. The vision was for a process-driven cybersecurity certification embedded within the Mindray development culture. This led us to the UL Solutions Cybersecurity Assurance Program,” said Frank Menzel, Program Director of System Solutions at Mindray North America.”
The UL 2900-2-1 certification assesses the security of products and systems that connect to networks, along with the processes used by vendors to develop and maintain these products, with a strong emphasis on security. UL Solutions CAP offers a comprehensive range of solutions aimed at assisting organizations in handling cybersecurity risks and demonstrating their cybersecurity capabilities to the market.
Receiving this certification underscores Mindray’s commitment to providing best-in-class solutions and demonstrating its products meet or exceed industry-standard cybersecurity measures.
“Mindray is excited about the combination of device testing and a secure by design process validation backed by the UL Solutions certification while also aligning with the NIST cybersecurity framework and UL 2900-2-1 being an FDA consensus standard for medical device cybersecurity,” said Menzel.
During the healthcare organization’s pre-purchase product qualification process, the UL Solutions certification provides customers with confidence that Mindray’s BeneVision N12, N15, and N17 patient monitors can easily fit into the healthcare system's existing cybersecurity program.
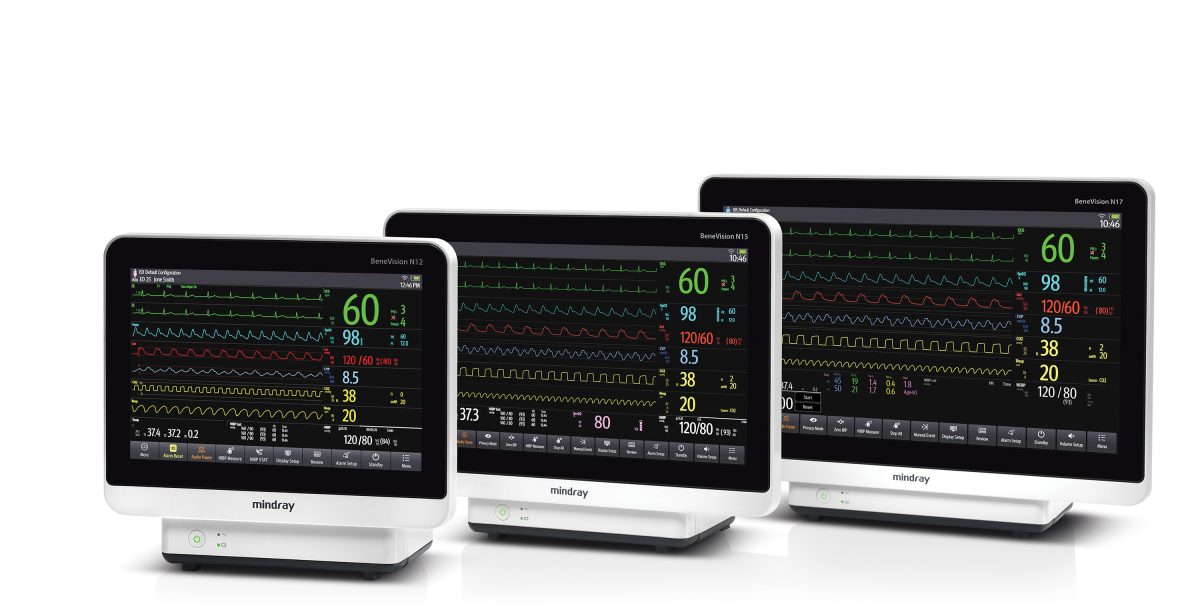
Mindray’s BeneVision N12, N15, and N17 patient monitors empower healthcare providers by offering a premier solution to satisfy patient monitoring needs across diverse care settings, including OR, ICU, NICU, and ER/Trauma. With a platform-wide modular design, expansive parameter options, and specialized Clinical Assistance Applications (CAAs), the N12, N15, and N17 patient monitors support clinical excellence and align nicely with organizational cost of ownership goals.
About Mindray
Mindray is a leading developer, manufacturer, and supplier of medical device solutions and technologies used in healthcare facilities worldwide. At Mindray, we rise up to meet the highest standards possible because we believe everyone deserves access to quality healthcare. As a result, we have been advancing medical technologies for more than 30 years providing robust, best-in-class solutions that are focused on our customers and designed to address their biggest pain points. Mindray offers innovative, leading-edge, accessible patient monitoring, anesthesia, and ultrasound solutions, empowering healthcare professionals to provide the highest quality of care now and in the future. For more information, please visit https://www.mindray.com/na.

