Graves’ disease is the most common cause of hyperthyroidism. Since TRAb (thyrotropin receptor antibody) is the pathogenic antibody of Graves’ disease, TRAb is considered to be one of the most important serum biomarkers for diagnosis, differential diagnosis, treatment monitoring and prognosis of Graves’ disease[1]. To provide a total thyroid solution for customers, Mindray spent five years on patented antibody authorization, reagent performance optimization and clinical validation, and now, we launch the TRAb assay to the global market.
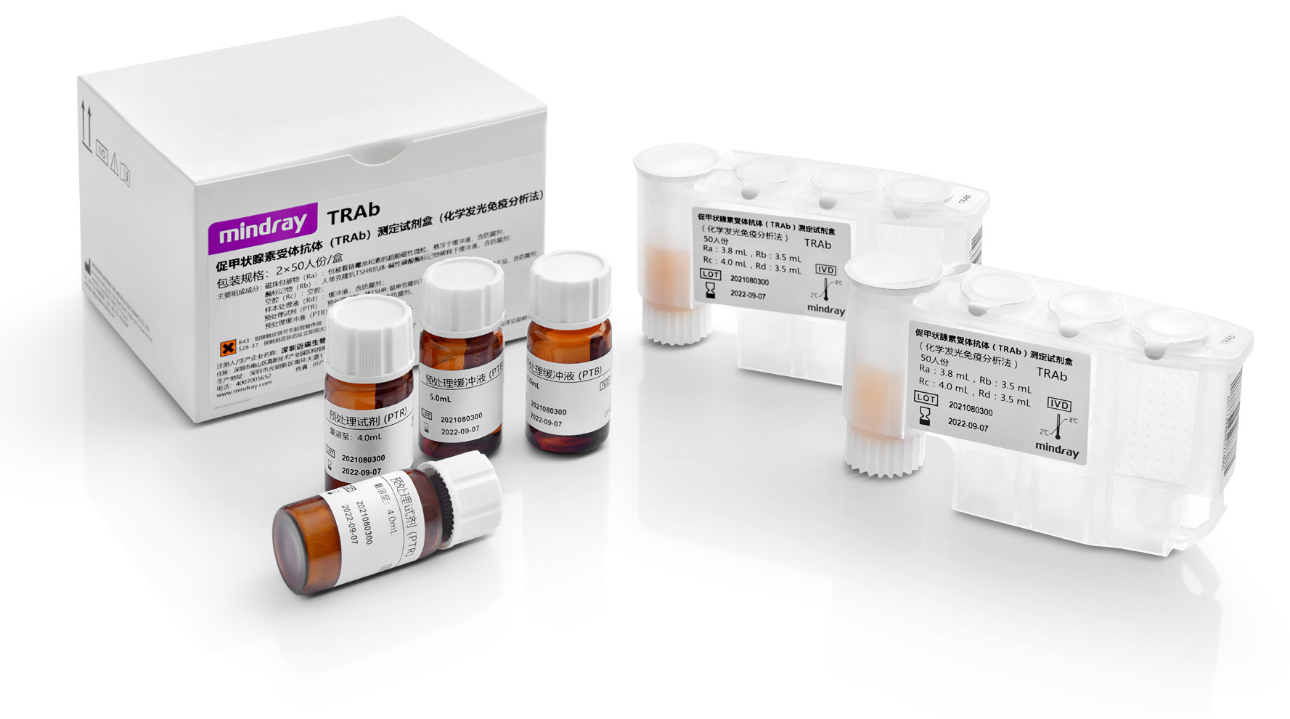
Preferred raw materials for reagents
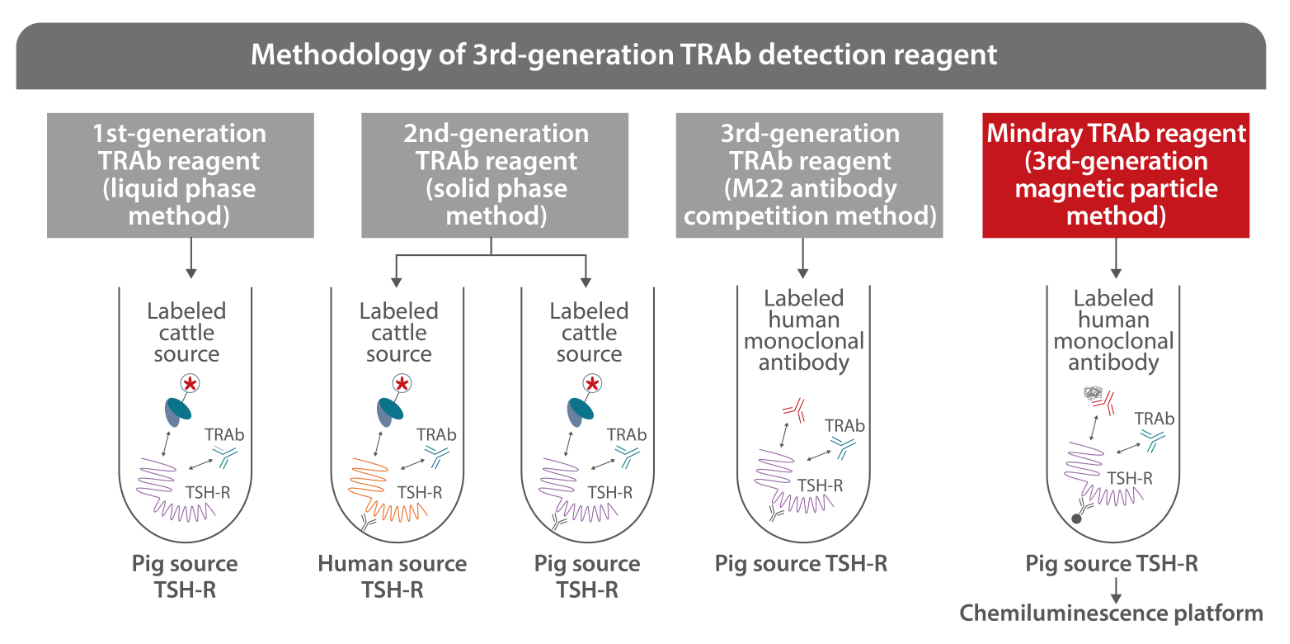
Mindray’s TRAb reagent is produced by a specific antibody – human monoclonal antibody M22, so as to improve the specificity and sensitivity of tests. The chemiluminescence platform allows the detection to be performed automatically with much improved efficiency.
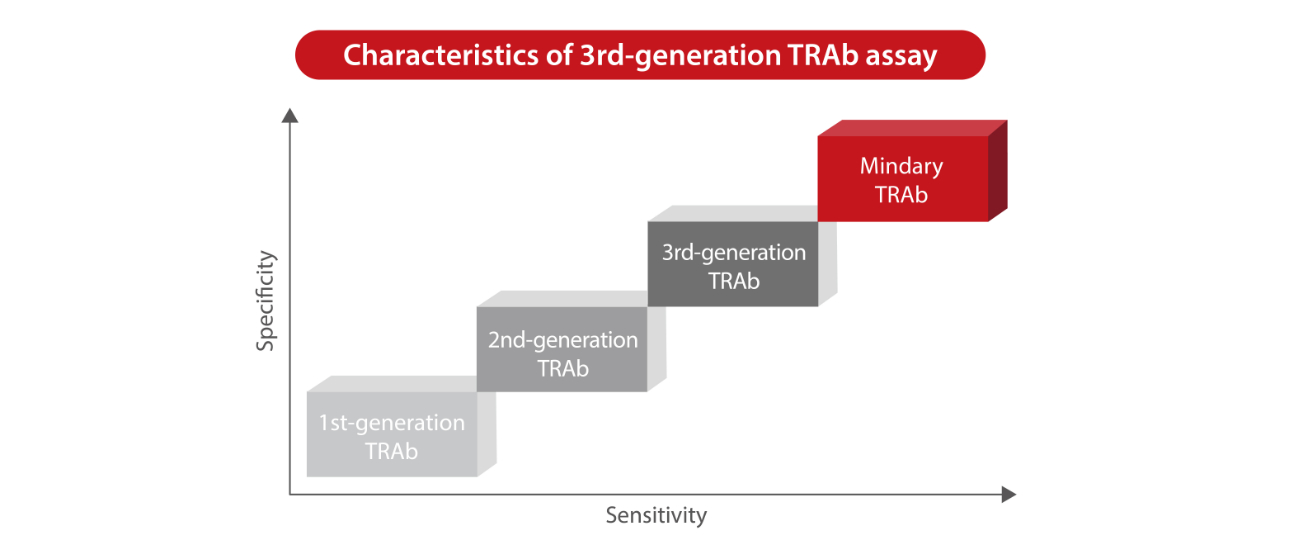
In 1956, the presence of "thyroid stimulating factor" in the serum of hyperthyroidism patients was demonstrated for the first time. In 1974, the 1st-generation TRAb detection reagent was made available. After decades of evolution and optimization, human monoclonal antibody M22 has emerged, which represents from liquid phase to solid phase detection and from animal derived antibody to humanized antibody, to bring revolutionary improvement to the detection of TRAb[2]. Mindray's TRAb assay adopts the 1st-generation TRAb detection reagent, which is upgraded and optimized on this basis. Its detection sensitivity, specificity and repeatability take the lead in the industry, providing accurate evidence for the diagnosis and treatment of Graves’ disease.
Reliable clinical performance
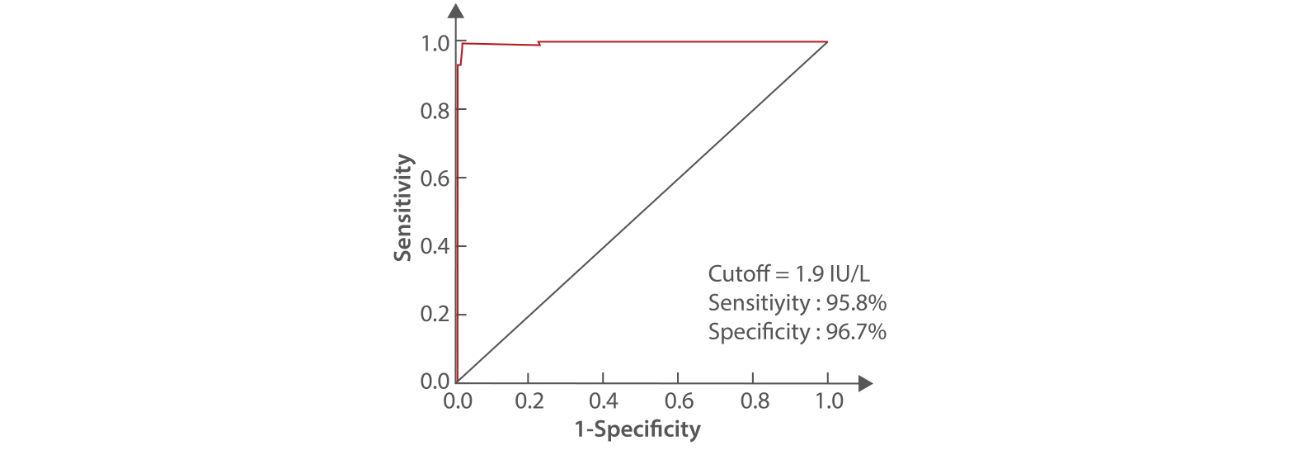
To ensure TRAb assay can meet all clinical requirements, Mindray thoroughly evaluated the clinical coincidence rate of TRAb assay before it was launched. Excellent results were achieved, showing Mindray TRAb assay has good sensitivity and specificity and is suitable for the diagnosis and differential diagnosis of Graves’ disease.
ROC curve represents the results when cutoff value is 1.9 IU/L. The sensitivity of Graves’ disease diagnosis is 95.8% and the specificity is 96.7%.
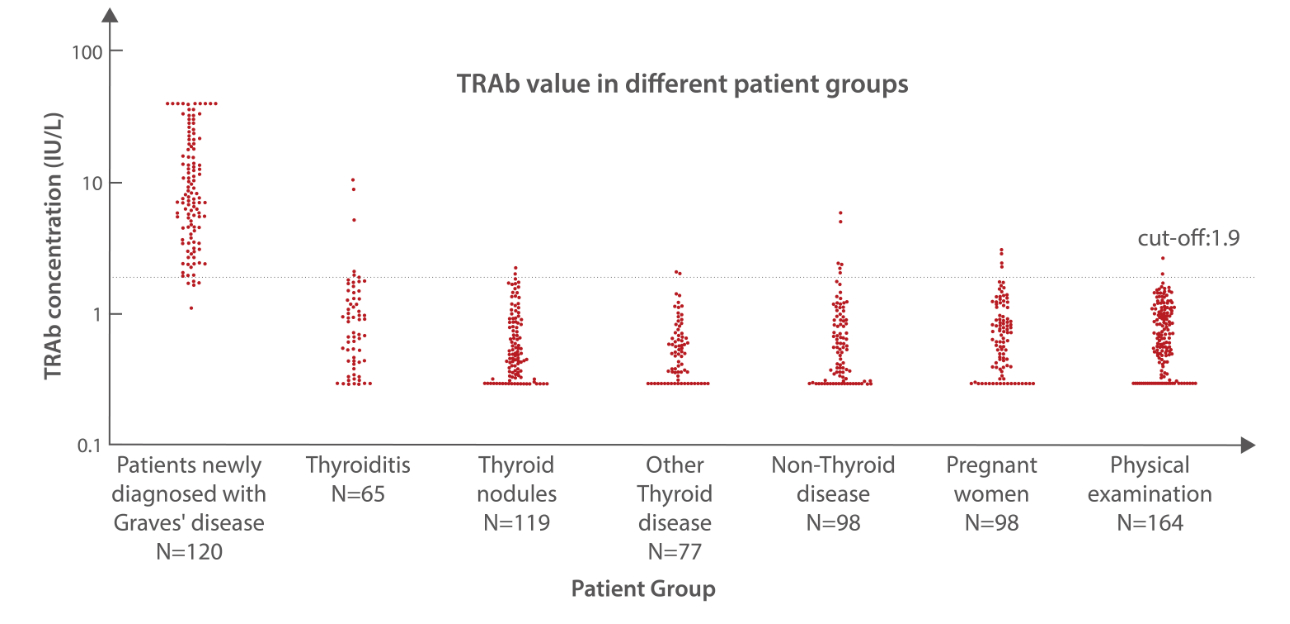
According to the concentration distribution of TRAb in different diseases, the concentration of serum TRAb is significantly higher in patients newly diagnosed with Graves’ disease than that in other patient groups, which means Mindray TRAb assay is suitable for the diagnosis and differential diagnosis Graves’ disease.
References
1. Clin J Perinat Med, Aug. 2019, Vol.22, No. 8., Guidelines for diagnosis and treatment of thyroid diseases in pregnancy and postpartum, Second Version.
2. Klaus Zophel, et al. Clinical review about TRAb assays’s History. Autoimmunity Reviews 9 (2010)695-700.