Hyperprolactinemia and Detection Challenges
Hyperprolactinemia, as a common endocrine disorder, has diverse clinical manifestations, including galactorrhea, menstrual irregularities, infertility, and compressive symptoms caused by pituitary tumors, among others [1,2].
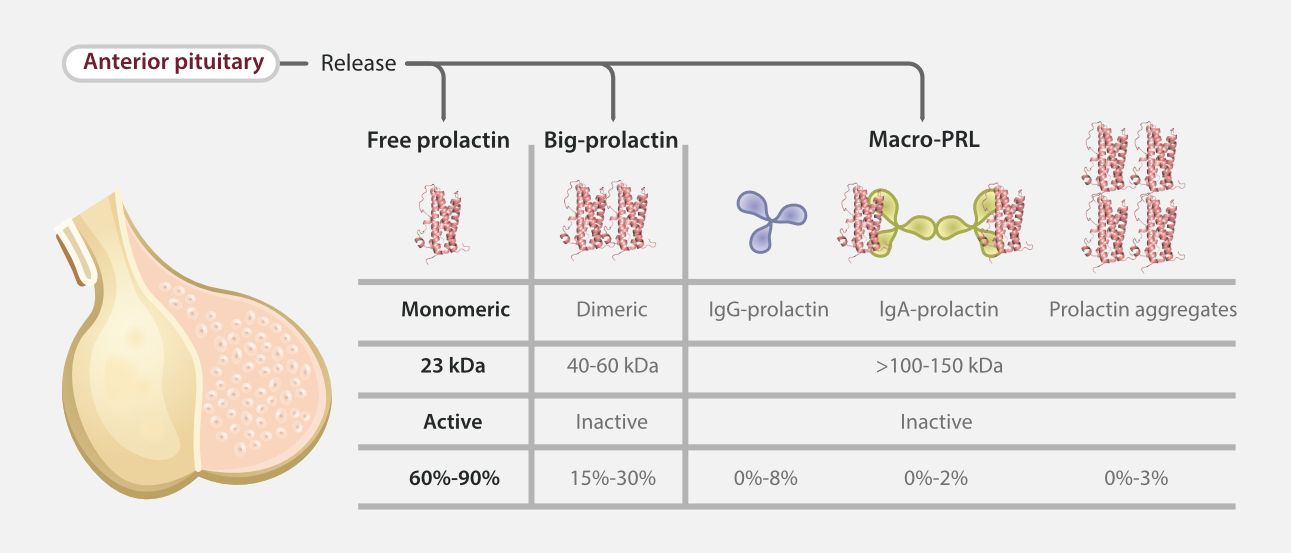
However, due to the existence of macroprolactin, traditional detection methods tend to misdiagnose macro-prolactinemia as hyperprolactinemia, leading to clinical harms such as excessive examinations, inappropriate drug treatments, and surgical interventions. Statistics show that the prevalence of macro-prolactinemia among the global hyperprolactinemia population is as high as 18.9%, highlighting the urgency of improving detection technologies [1,2].
Mindray's Groundbreaking Innovation in PRL Detection Technology
In the face of this challenge, Mindray Medical, in collaboration with scientists from Hytest, has jointly developed a new type of PRL reagent. By computationally analyzing the structural characteristics of PRL and its autoimmune epitopes, the reagent identifies the differential sites between monomeric prolactin and macroprolactin. It then conducts directional immunization and screens out antibodies that bind with high affinity to monomeric prolactin but do not bind to macroprolactin, thereby achieving strong resistance to interference from macroprolactin.
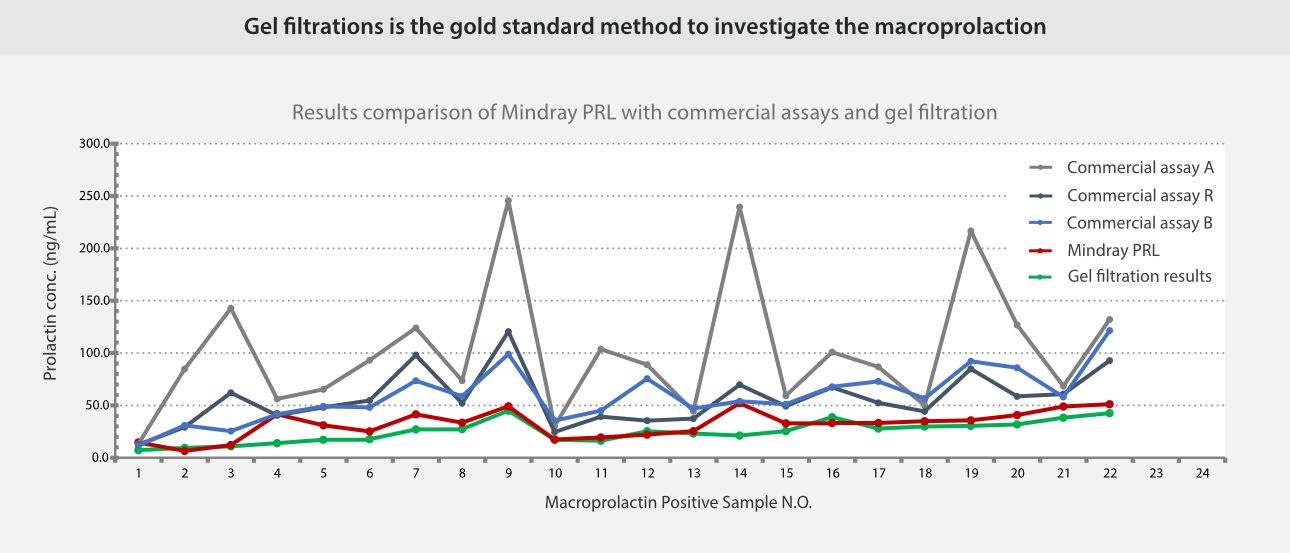
Real-world data indicates that in macroprolactin-positive samples, Mindray's PRL measurements strongly correlate with gel filtration chromatography results, while in true hyperprolactinemia samples, the detection results align with those of other systems, validating the accuracy and reliability of the new PRL assay.
References
[1] Lippi G, Plebani M. Macroprolactin: searching for a needle in a haystack?. Clin Chem Lab Med. 2016;54(4):519-522. doi:10.1515/cclm-2015-1283
[2] Cozzi R, Ambrosio MR, Attanasio R, et al. Italian Association of Clinical Endocrinologists (AME) and International Chapter of Clinical Endocrinology (ICCE). Position statement for clinical practice: prolactin-secreting tumors. Eur J Endocrinol. 2022;186(3):P1-P33. Published 2022 Feb 3. doi:10.1530/EJE-21-0977