High sensitivity and specificity
To achieve high sensitivity and specificity, Mindray has been working consistently to improve the raw materials, processes, formulas, and reaction models. High quality materials are fundamental to ensure high sensitivity and specificity of CLIA assays. To cater to different projects and process requirements, Mindray invested a lot of resources in the design and development of immunodominant proteins and immunodominant epitopes.
Efficient processes
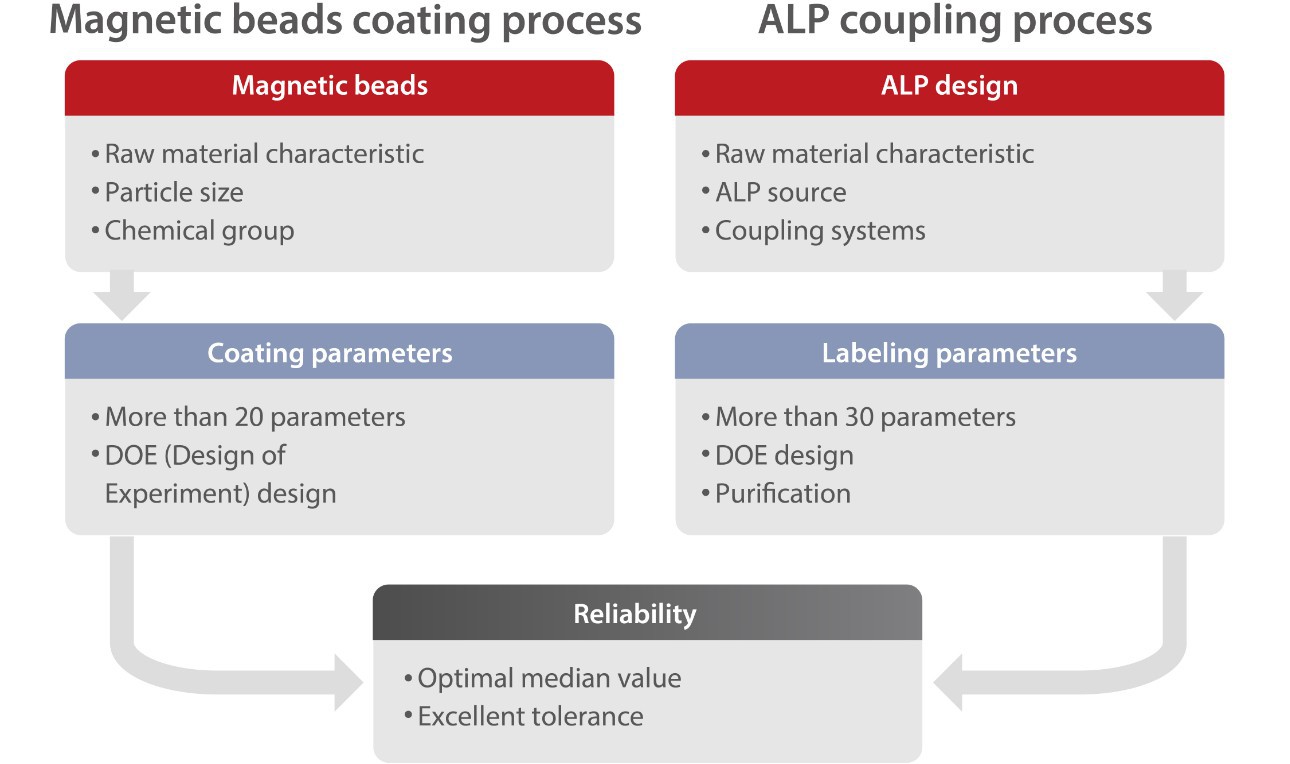
Mature, stable and innovative technology is another guarantee of satisfactory performance. For the coating and labeling processes, Mindray R&D team selected the particles and ALPs that can best meet the assay requirements. To improve the sensitivity and specificity, Mindray experts spent a considerable amount of time thoroughly studying the parameters using a DOE approach. With a robust design, the boundary of each parameter was determined to ensure the reproducibility of the process.
Advanced formulas and reaction models
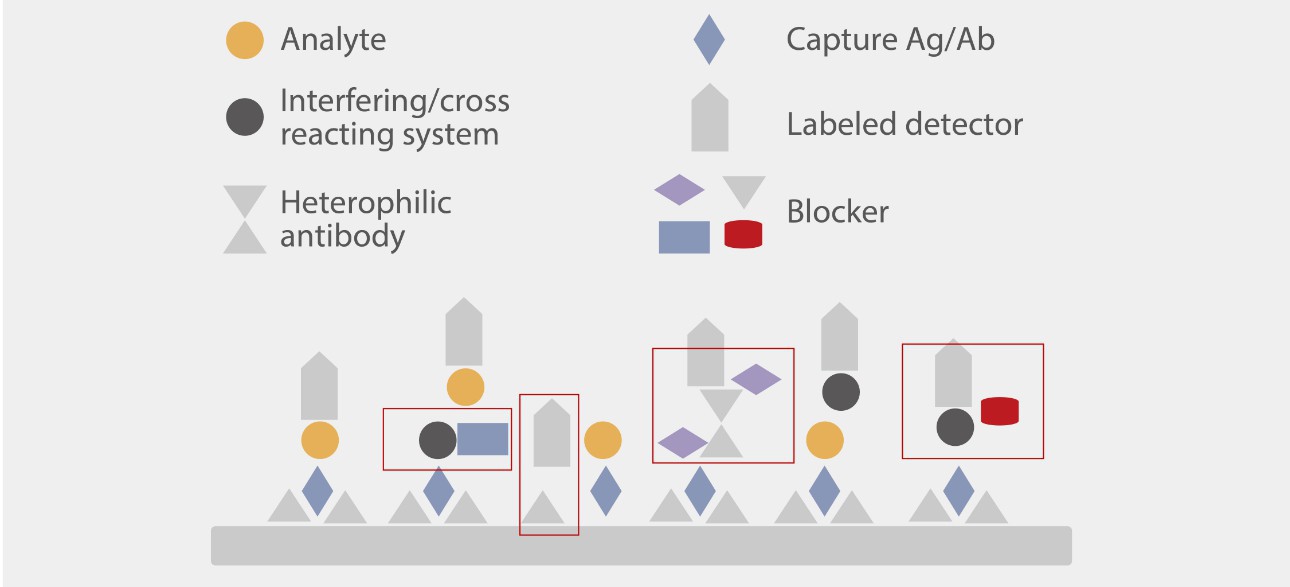
At the formulation level, Mindray R&D experts investigated the interference mechanism and classified the interference into four different types to help enhance the anti-interference capacity.
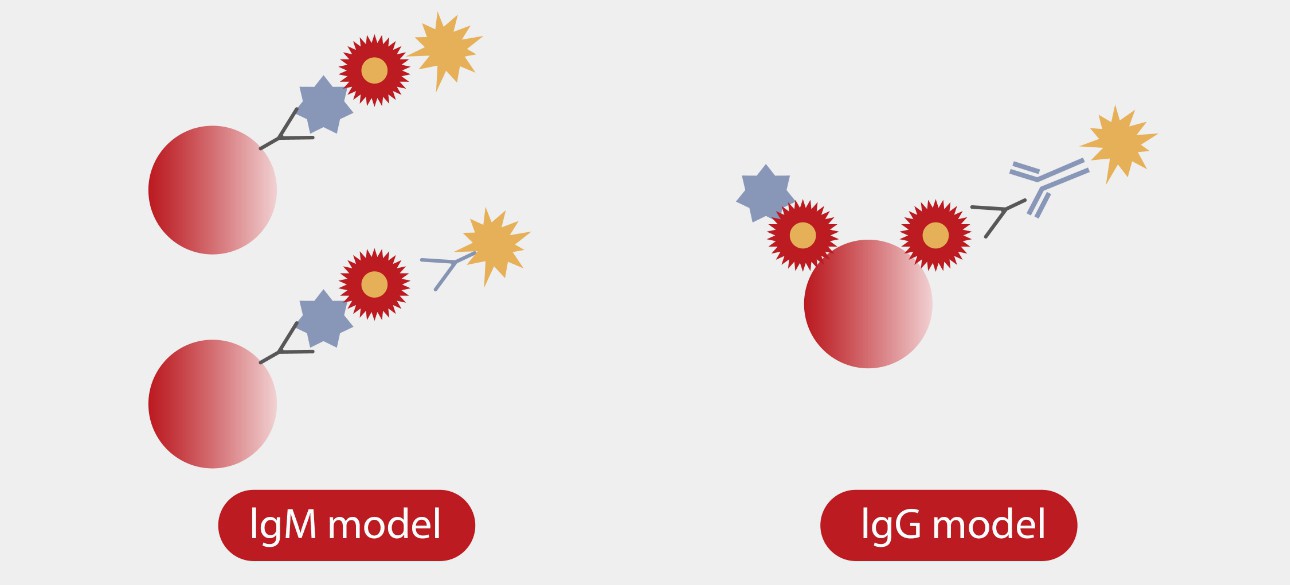
Hundreds of commercial and uniquely designed blockers were studied and applied to minimize each type of interference. For instance, Mindray R&D team designed a capture method for the IgM assay to minimize the interference of IgG and RF (Rheumatoid Factor).
Traceability of IgG for reliable medical determination

The above three quantitative kits of Mindray ToRCH panel are fabricated with materials with international standard units and the latest standardized traceability. Toxo IgG contains specific IgG and features DT test standardization. CMV IgG adopts the first international standard and features long-term stability. RV IgG comes with a cutoff value of 10 IU/mL as recommended by WHO.
Seroconversion sensitivity[2]
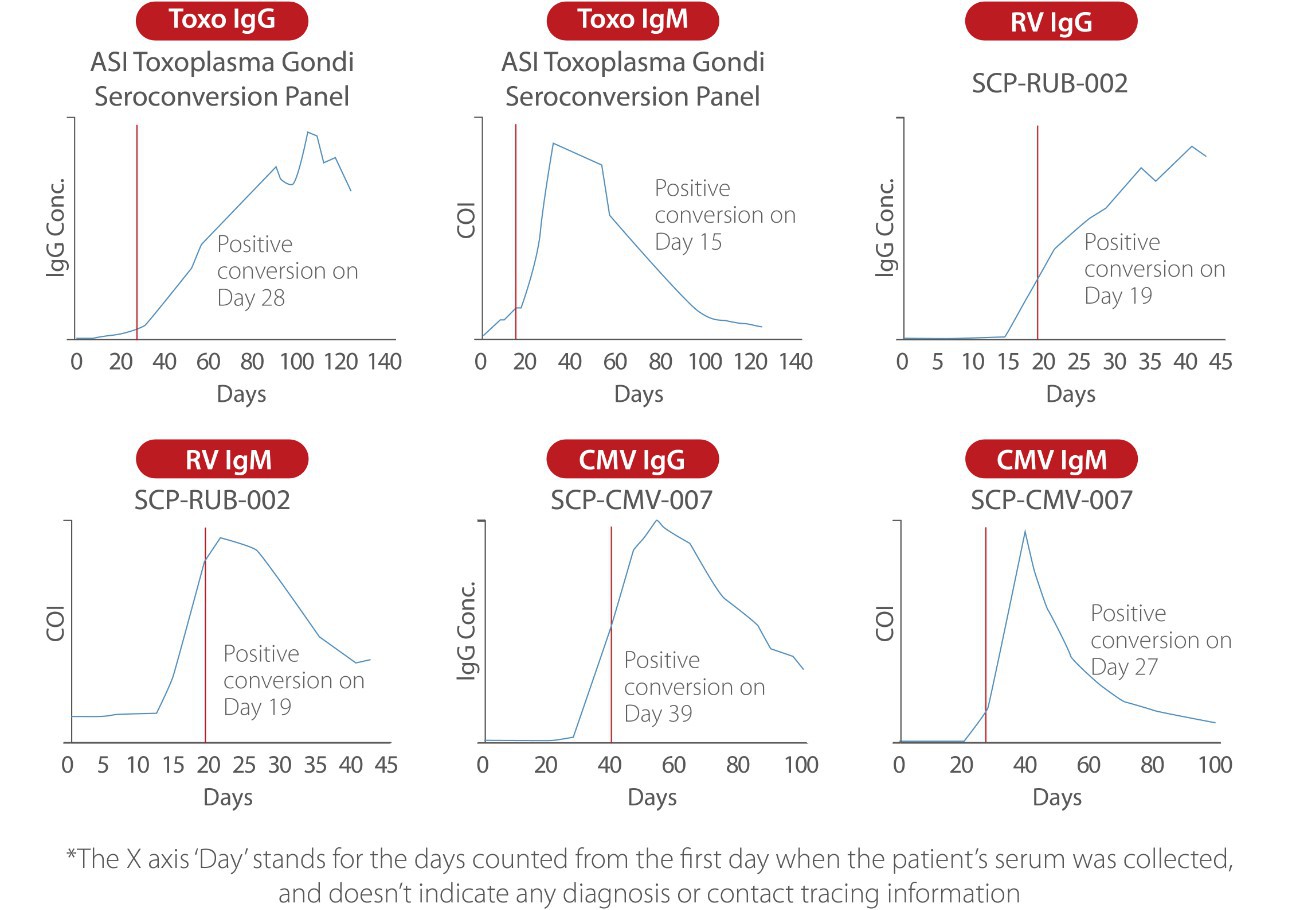
Thanks to the improved raw materials, processes, formulas, reaction models, and standardized traceability, Mindray ToRCH reagents deliver excellent seroconversion sensitivity. Mindray ToRCH has a good detection rate for both samples that are positive in multiple systems and true positive samples such as seroconversion panels. The combined examination by Mindray ToRCH IgG and IgM can accurately reveal previous infections and promptly detect ToRCH infections that failed to be ruled out previously.
Excellent clinical performance
To demonstrate the sensitivity and specificity of the Mindray ToRCH kits, Mindray conducted tests in over five clinical sites both in and outside China.
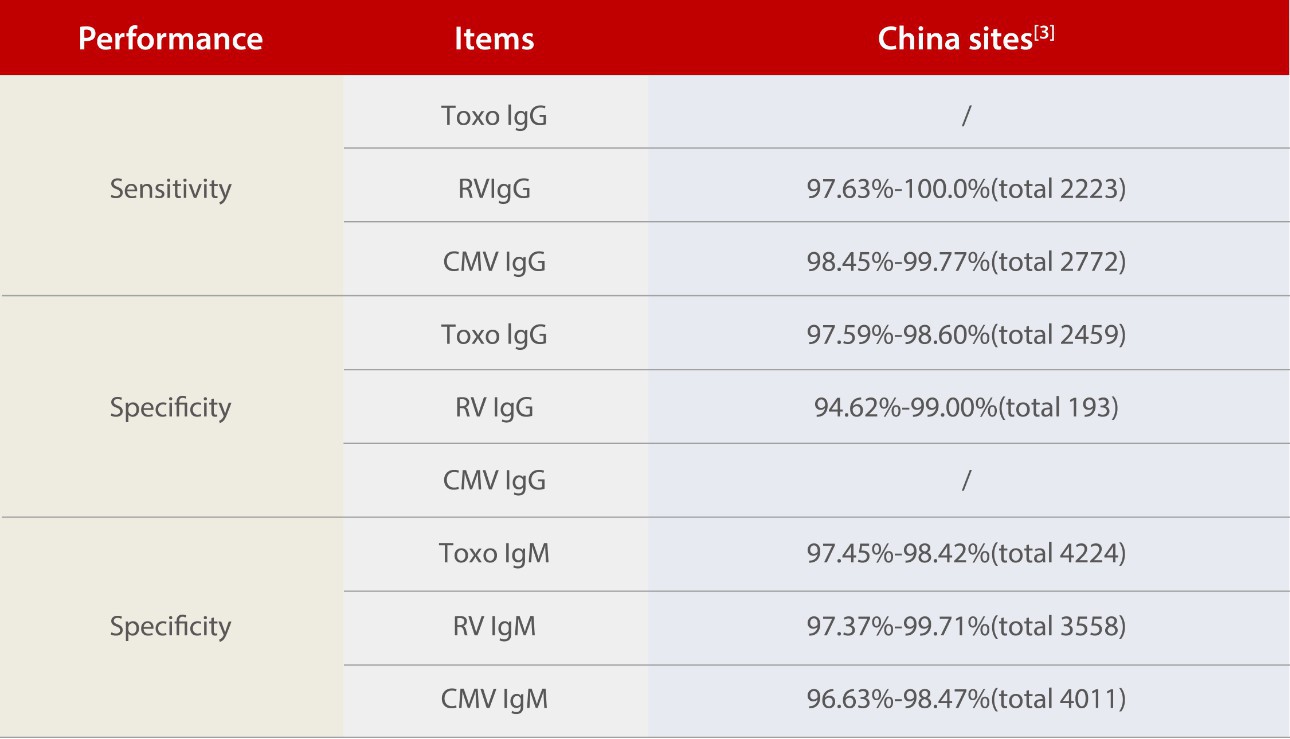
Although the positive rate varies among different countries and regions, Mindray ToRCH kits have exhibited great specificity for Toxo IgG and ToRCH IgM and excellent sensitivity for RV IgG and CMV IgG. In addition, given the wide coverage of the clinical tests, there are no significant differences between the Chinese and international sites.
Flexible blood collection and transportation
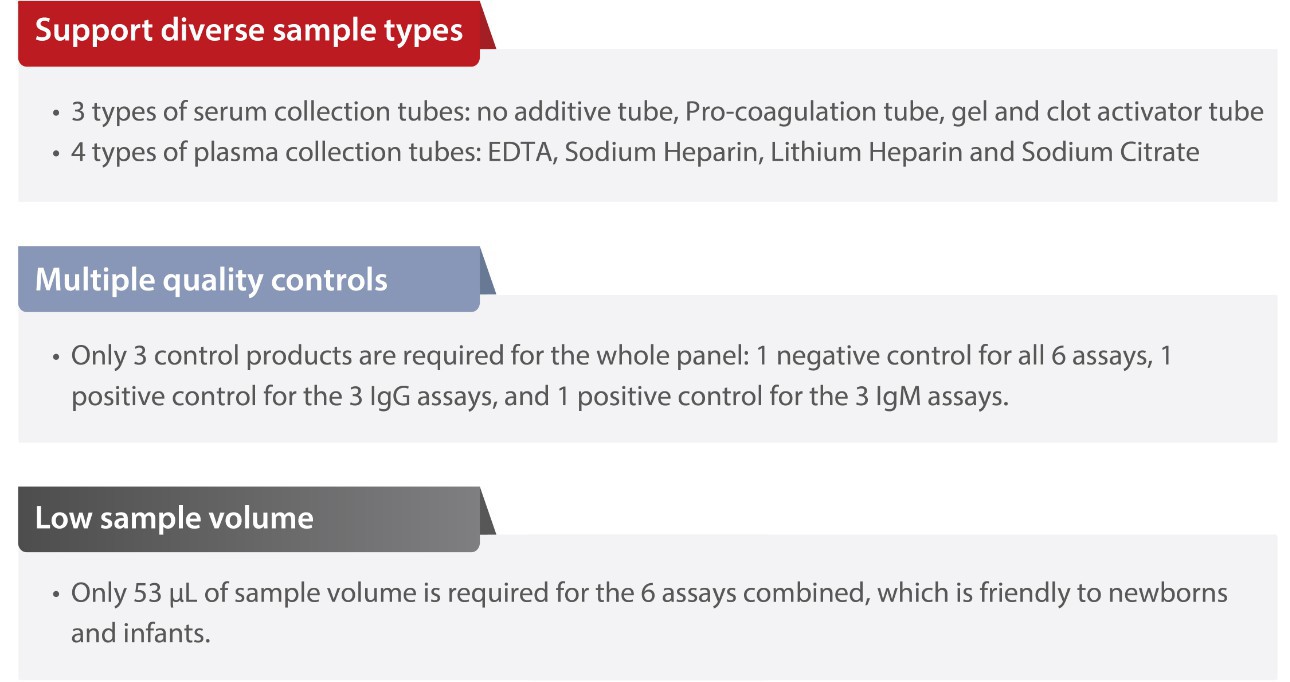
Mindray ToRCH kits support diverse sample types with less quality controls and sample volumes, which ensures great ease and convenience during clinical detection.
By bringing the clinical benefits of ToRCH Panel into play and addressing the key requirements for the reagents, Mindray has solved the ToRCH detection challenges facing clinical laboratories and made Mindray ToRCH assays accessible in clinical application.
References
[1] Mindray ToRCH IFU
[2] Mindray internal evaluation
[3] Mindray external evaluation

