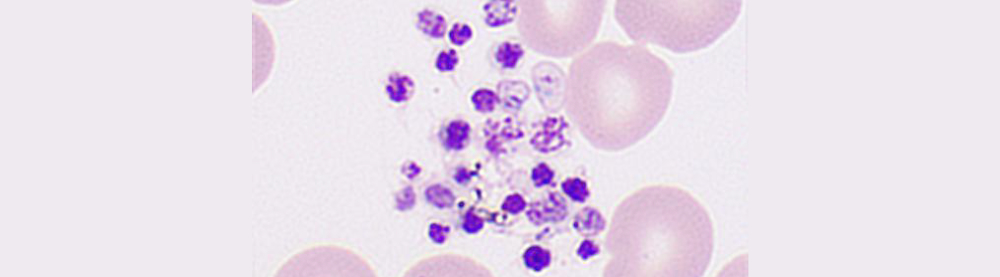
Ethylenediaminetetraacetic Acid - Pseudothrombocytopenia (EDTA-PTCP) is a laboratory artifact that may lead to an incorrect evaluation and unnecessary treatment of patients.
Which of the following method (or methods) would you take to correct platelet counts in case of an EDTA-induced platelet aggregation in thrombocytopenia?
- Recheck with blood smear and estimate the platelet count
- Recheck with addition of amikacin
- Recheck after warming at 37°C
- Recheck immediately dilution without any anticoagulant
- Reexamine on another hematology device
- Recheck with other anticoagulants
Professional clinical laboratory doctors pursue accuracy and truth with the highest sense of responsibility. Nowadays, some clinical studies have been conducted to explore EDTA-PTCP solutions, and suggesting that Mindray hematology systems with SF Cube technology would be an option to effectively assist lab technicians in identifying correct platelet counts.

A Clinical Case Report
The patient here is a 32-year-old female with infertility. After the patient’ s EDTA-anticoagulated blood was drawn, it was analyzed within fifty-five minutes, and it showed a low platelet count (28 × 109/L). The test was done by the impedance method (PLT-I) on a popular brand’ s hematology system (device A). Platelet aggregation was confirmed by microscopic examination of the smear, indicating pseudothrombocytopenia (PTCP). Shortly after, a reexamination of this sample was done using the CDR(PLT-O) method on the Mindray BC-6800Plus. The results showed a markedly higher platelet count with a value of 180 × 109/L.
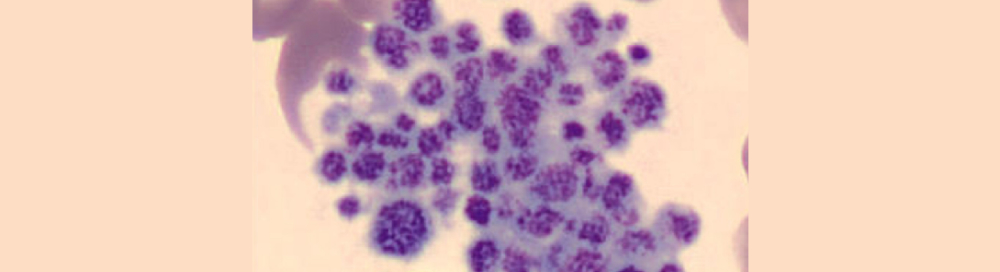
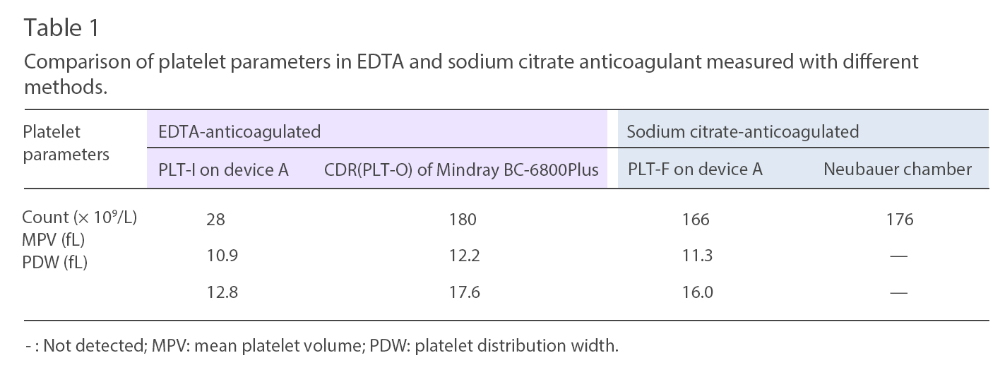
It’s suspected that the low platelet count obtained by device A was due to EDTA-induced PTCP. So, the patient was asked to consent for an additional test using a blood sample tube with sodium citrate this time. Thirty minutes after the sample was collected it was then analyzed. The resulting platelet parameters of the blood samples run through various testing methods and devices are listed in Table 1.
Further Comparison of Other Samples
Under microscopic evaluation of the blood smear, the EDTA-anticoagulated blood showed platelet aggregation while the sodium citrate-anticoagulated blood showed none. The blood samples were analyzed within four hours from the time of collection, according to the manufacturer's instructions. In addition to that patient, the data from an additional five cases of EDTA-PTCP were collected and assessed (Table 2).
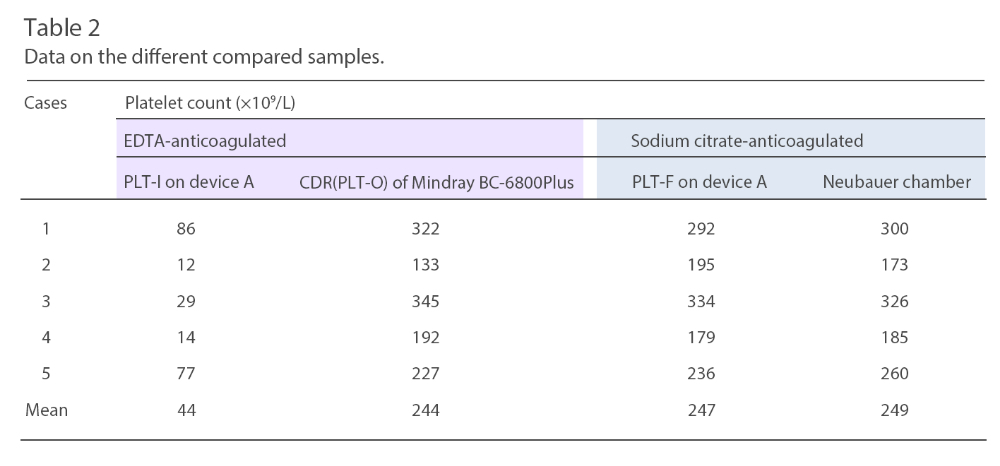
In the end, the author concludes: In patients with known or suspected EDTA-PTCP Mindray SF-Cube technology is a straightforward and effective way of determining the platelet count in EDTA-anticoagulated blood.

Identification and Characteristics of EDTA-PTCP Samples
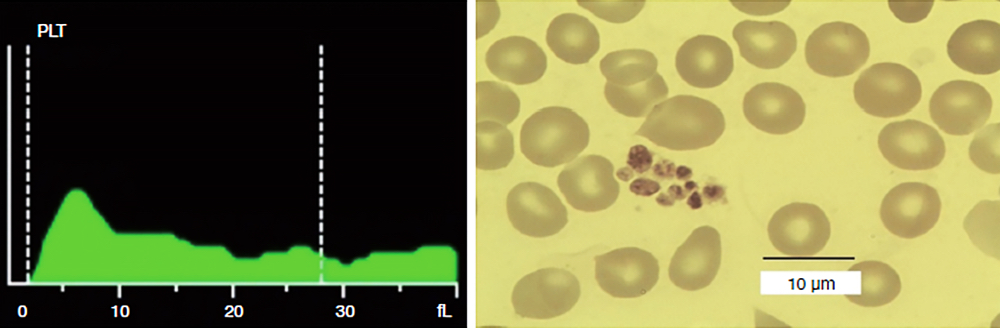
Samples that triggered the “PLT aggregation” flag on the hematology analyzer showed a typical serrated irregularity and a zigzag tail (Figure 1) on the platelet histogram. Also, under microscopic evaluation, the presence of platelet satellitism, or giant platelets, is not seen.
Spurious Low Platelet Counts of BC-6800 by Optical Platelet Counting (PLT-O)
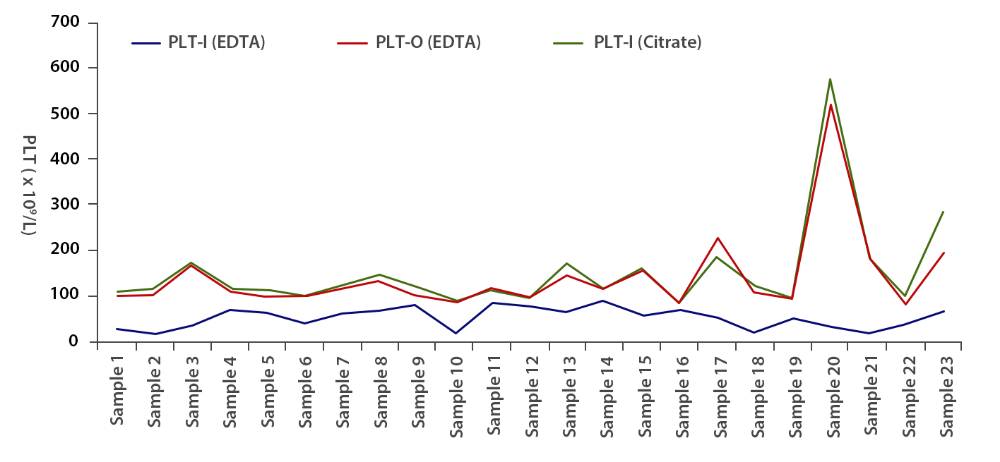
Twenty-three EDTA-PTCP samples in EDTA tubes (with platelet aggregation) were tested in the impedence (PLT-I (EDTA)) and reticulocyte channel of the Mindray BC-6800 (PLT-O (EDTA)). Interestingly, the PLT-O (EDTA) results were comparable to) the platelet counts of the re-collected samples in citrate tubes (PLT-I (Citrate)), which proves that the PLT-O (EDTA) method can accurately adjust for the platelet aggregation issue caused by EDTA (Figure 2).
One Mainstream Brand’s Hematology System’s EDTA-PTCP Dissociation Effect:Available with Fluorescent Dye Staining?
Optical fluorescence platelet counting is available in both high-end hematology analyzers (device B) and Mindray BC-6000 series hematology analyzers. In this method, a fluorescent dye is used to stain the nucleic acids in platelets, allowing the recognition of large platelets and excluding non-platelet particles such as erythrocyte debris, micro erythrocytes, or leukocyte debris.
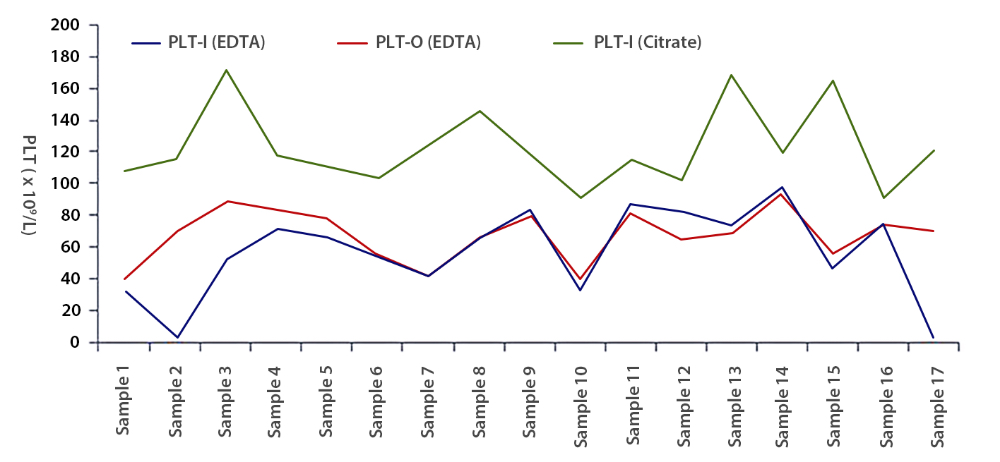
To verify whether the dissociation effect of optical fluorescence platelet counting was dependent on fluorescent dye staining, 17 of those 23 EDTA-PTCP samples in EDTA tubes were also tested on device B's reticulocyte channel and impedance channel., It was found that here was no significant difference between the platelet counts of reticulocyte channel and impedance channel (Figure 3). Only one of the 17 EDTA-PTCP samples showed a dissociation rate of more than 80%, with an average dissociation rate of 56% among all 17 EDTA-PTCP samples (Figure 3).
Conclusion
In summary, the author concludes: Optical fluorescence platelet counting of the BC-6800 hematology analyzer is effective for the correction of spurious low platelet counts in EDTA-PTCP patients, and its dissociation effect on EDTA-PTCP samples is independent of fluorescent dye staining.
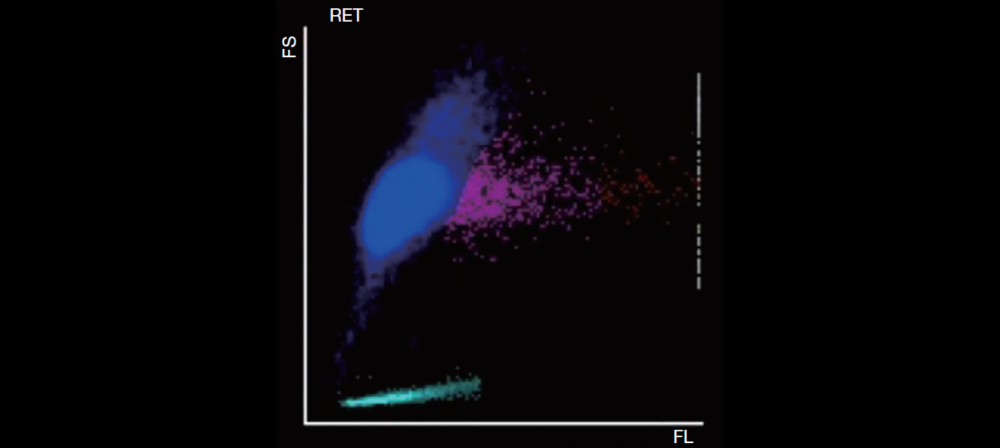
In the busy daily work of the laboratory, PTCP is an inevitable trouble. Mindray SF Cube technology provides PLT-O (based on nucleic acid fluorescent staining and done on the RET channel) to correct PLT counts when there is pseudo-platelet reduction due to EDTA. PLT-O is available on Mindray BC-6000 Series Auto Hematology Analyzer and the CAL 8000/6000 Cellular Analysis Line.
References:
[1] J. Deng, et al. Mindray SF-Cube technology: An effective way for correcting platelet count in individuals with EDTA dependent pseudo thrombocytopenia. Clinica Chimica Acta 502 (2020) 99–101
[2] Y. Bao, et al. Correction of spurious low platelet counts by optical fluorescence platelet counting of BC-6800 hematology analyzer in EDTA-dependent pseudo thrombocytopenia patients. Transl Cancer Res 2020;9(1):166-172