HemaBook Chapter 26: HMC Channel: Accurate Identification of Abnormal WBCs in Peripheral Blood
2025-08-07

The Significance of HMC Channel
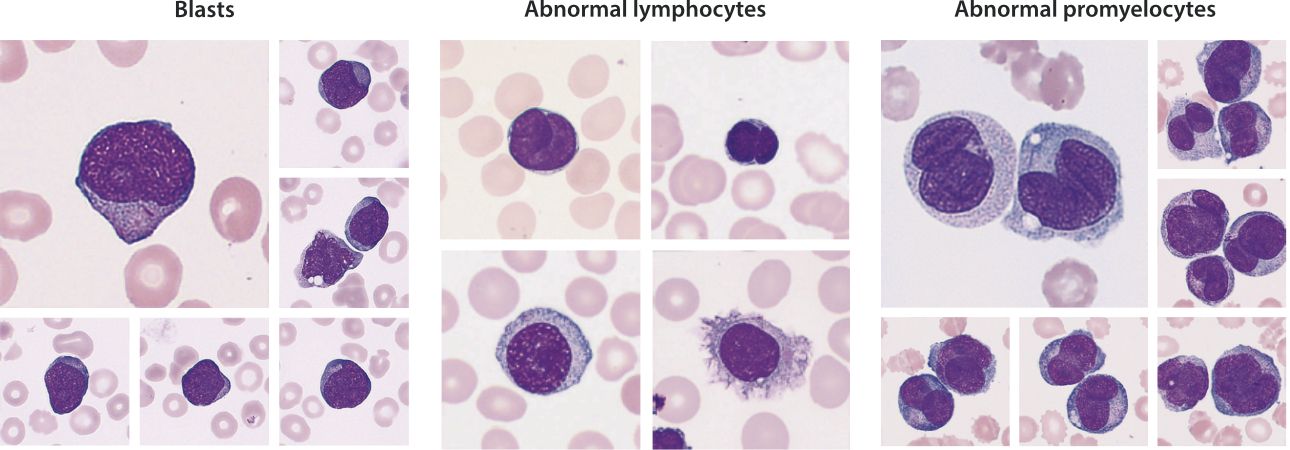
Hematological malignancy is one of the most common categories of cancer [1]. When the human body is affected by hematological malignancies, hematological malignant cells (such as blasts, abnormal promyelocytes and abnormal lymphocytes, etc.) may appear in peripheral blood. Therefore, accurate detection of hematological malignant cells in peripheral blood is of great significance for the early screening, disease monitoring, and prognosis assessment of hematological malignancies.
Mindray's automated hematology analyzer integrates the innovative SF Cube technology platform and the newly launched Hematological Malignant Cells (HMC) channel. Consequently, it achieves precise detection of blasts, abnormal promyelocytes and abnormal lymphocytes. This provides strong support for the screening of hematological malignancies. The mechanism of HMC channel is illustrated in this chapter.
The Mechanism of HMC Channel
Given that blast cells, abnormal lymphocytes, and abnormal promyelocytes have significant differences from other cells in three key dimensions [2-4]: the proportion of the phospholipid in the cell membrane, the spatial complexity of the nuclear DNA, and the complexity of the cytoplasmic internal structure, we have carefully designed a reagent reaction system based on specific principles.
Firstly, cells react with LM lysing agent. When the LM lysing agent acts on the cell membrane, it will dissolve the phospholipid within the membrane, and then enter the cell, causing differential changes in the optical refractive index of various cells. Blasts have a high percentage of phospholipid in their cell membranes, and after treatment, a large amount of LM lysing enters the blasts, resulting in a decrease in their optical refractive index. Since the level of the optical refractive index of the cell will have an effect on the strength of the forward scattered light signal, the detection and identification of blasts can be realized with the help of the difference of the forward scattered light signal between various types of cells.
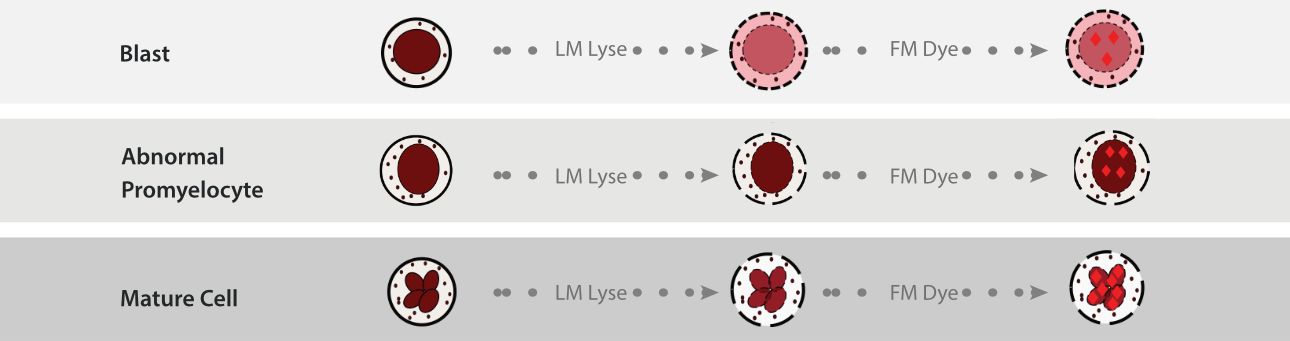
After LM treatment, the FM fluorescent dye enters into the nucleus. The chemical structure of the FM dye can specifically bind to the double-stranded DNA in the cell nucleus through an intercalation mechanism. Among them, the DNA spatial structure of blast cells and abnormal promyelocytes is more complex, with fewer binding sites for the dye, resulting in lower fluorescence intensity. In contrast, the DNA spatial structure of abnormal lymphocytes is relatively loose, thus exhibiting a high-intensity fluorescence signal. Herein, fluoresce signals with different strengths are generated.
Notably, the complexity of the cytoplasmic internal structure cell remains intact even after the LM and FM treatment, which means the side scatter light signal could still reveal the original cytoplasmic internal complexity of the cells. Thus, combined with the SF Cube three-dimensional analysis algorithm, it successfully achieves precise identification and detection of blast cells, abnormal promyelocytes, and abnormal lymphocytes by capturing and analyzing forward scatter (FS), fluorescence signals (FL), and side scatter (SS), providing key clues for disease screening.
| Cell type | Degree of lysing | Degree of fluorescent dye bind | FS | SS | FL |
|---|---|---|---|---|---|
| Blast | Strong | Weak | Weak~Medium | Weak | Weak~Medium |
| Lymphocyte & abnormal lymphocyte | Strong | Medium~Strong | Weak~Medium | Weak | Medium~Strong |
| Abnormal Promyelocyte | Medium | Weak | Strong | Medium~Strong | Weak~Medium |
| Normal WBCs | Weak | Medium~Strong | Medium~Strong | Medium~Strong | Medium~Strong |
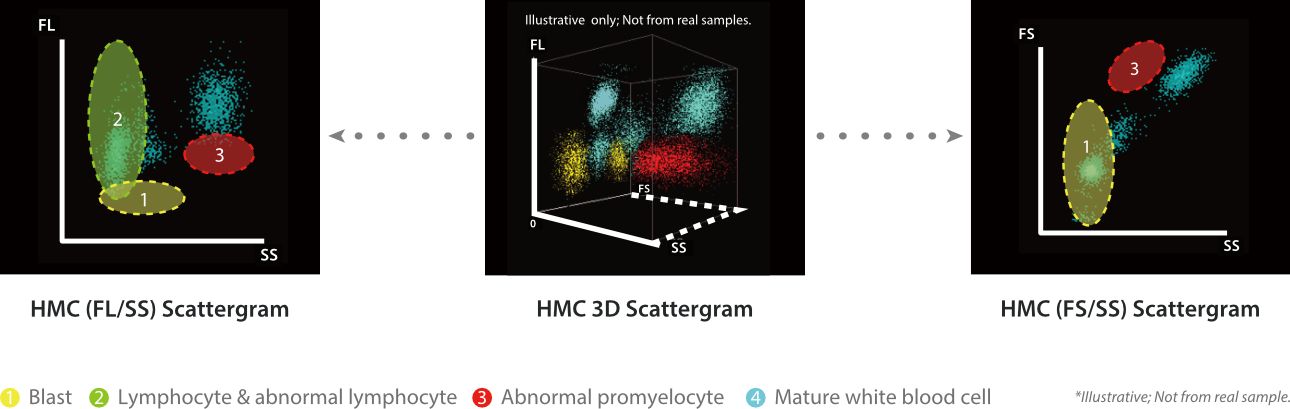
Conclusion
The HMC channel provides an efficient and reliable solution for accurate screening of hematological malignant cells in peripheral blood by innovatively combining LM and FM. In terms of clinical application, HMC channel can effectively detect hematological malignant cells in peripheral blood under different scenarios of blood diseases through many actual cases, providing key initial screening clues for subsequent diagnosis of the disease, assisting clinicians to make timely and accurate judgments, and formulating reasonable treatment plans, which plays a positive role in improving the prognosis of the patients and increasing the cure rate. More clinical cases will be illustrated in the next chapter.
References:
[1] Choi, H.-S., Kim, B. S., Yoon, S., Oh, S.-O., & Lee, D, Leukemic Stem Cells and Hematological Malignancies. International Journal of Molecular Sciences, 25(12)(2024), 6639.
[2] A.E. Fazary, Y.H. Ju, H.S.M. Abd-Rabboh, How does chromatin package DNA within nucleus and regulate gene expression?, International journal of biological macromolecules 101 (2017) 862-881.
[3] X. Chen, H. Lin, G. Li, The influence of high-order chromatin state in the regulation of stem cell fate, Biochemical Society transactions 50(6) (2022) 1809-1822.
[4] L. Scourzic, E. Salataj, E. Apostolou, Deciphering the Complexity of 3D Chromatin Organization Driving Lymphopoiesis and Lymphoid Malignancies, Frontiers in immunology 12 (2021) 669881.

