A Glimpse from EuroMedLab 2025: PRL Workshop Unveils Breakthroughs in Prolactin Detection
2025-05-27

At the 26th IFCC-EFLM EuroMedLab Congress of Clinical Chemistry and Laboratory Medicine held in Brussels, Belgium, from May 18 to 22, 2025, the PRL (Prolactin) Workshop stood out as a key focus, capturing the interest of experts and industry leaders in laboratory medicine from around the globe. The Workshop addressed the primary challenges, including a detailed look at macroprolactin interference, a key issue in diagnosing hyperprolactinemia, while featuring discussions on practical solutions to tackle these challenges. The Workshop also showcased Mindray’s latest technological breakthroughs made in prolactin detection.

Featured Experts and Their Pioneering Contributions
The Workshop welcomed Prof. Fahie-Wilson—a recognized expert with 28 years of experience in detecting macroprolactin interference—to share insights and lead discussions. As a leading authority in the field, Prof. Fahie-Wilson has published more than 20 influential papers and three comprehensive reviews. He also pioneered the polyethylene glycol (PEG) precipitation method capable of distinguishing macroprolactinemia from monomeric prolactin, which represents a major contribution to the precision of laboratory testing. At the event, Prof. Fahie-Wilson gave a thorough presentation on the excellent performance of Mindray’s new PRL assay. By modifying the antibody binding epitope, the assay offers strong resistance to macroprolactin interference, helping to enable precise diagnosis of hyperprolactinemia.
Hyperprolactinemia and Detection Challenges
Hyperprolactinemia, a common endocrine disorder, presents with a variety of clinical symptoms, including galactorrhea, menstrual irregularities, infertility, and pituitary tumor compression symptoms. However, the presence of macroprolactinemia—where macro-molecular-weight prolactin predominates in peripheral blood—often leads to misdiagnoses when traditional detection methods are used. This would bring about clinical hazards such as unnecessary tests, inappropriate medication treatments, and even improper surgical interventions. Statistics show that macroprolactinemia accounts for as high as 18.9% of global hyperprolactinemia cases, underscoring the pressing need to improve detection technologies.
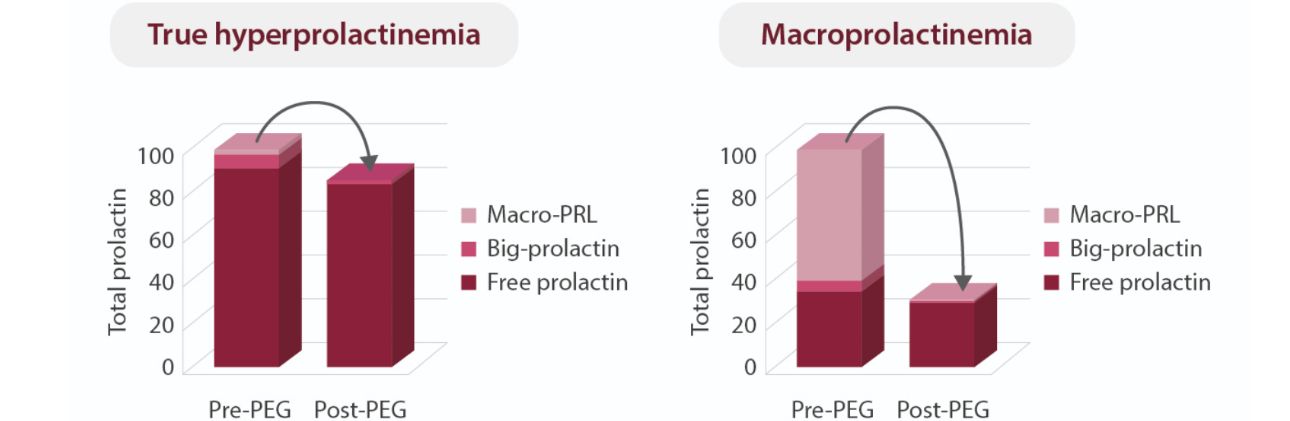
Mindray's Groundbreaking Innovation in PRL Detection Technology
Facing this challenge, Mindray worked with scientists from the Supercomputing AI Center and HyTest to develop a new PRL assay. The assay identifies aggregation hotspots and immunogenic epitopes, leveraging high-affinity monoclonal antibodies and AI tools to efficiently screen for antibodies targeting the desired epitopes. By utilizing protein folding prediction technology for modeling, it demonstrates strong resistance to macroprolactin interference.
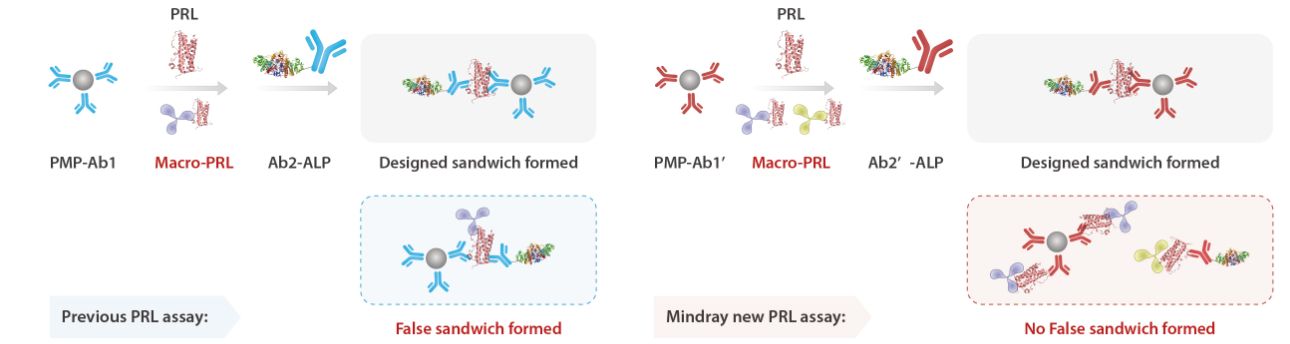
Real-world data indicates that in macroprolactin-positive samples, Mindray's PRL measurements strongly correlate with gel filtration chromatography results, while in true hyperprolactinemia samples, the detection results align with those of other systems, validating the accuracy and reliability of the new PRL assay.
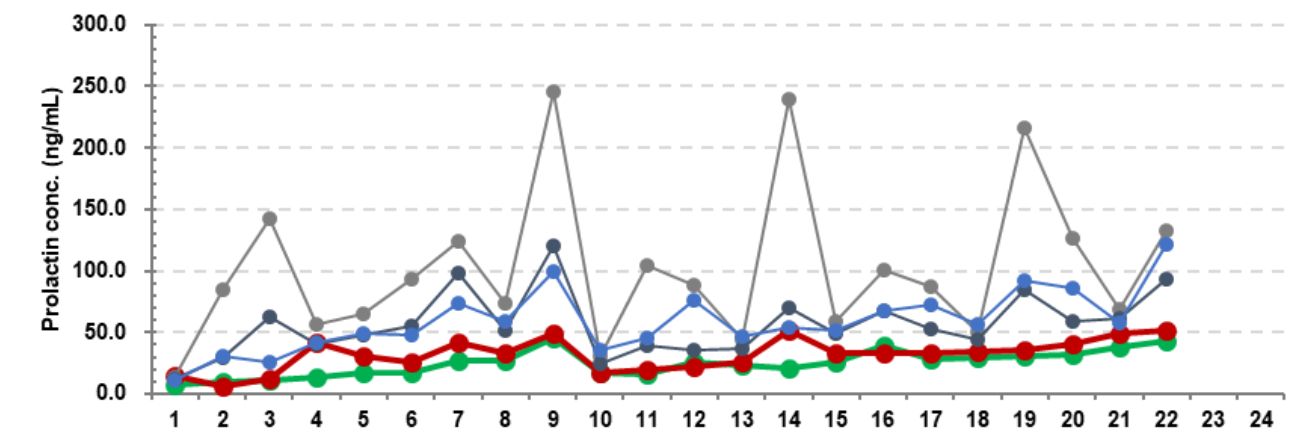
Takeaways and Outlook
At the PRL Workshop, presentations about Mindray's recent advancements in prolactin detection triggered profound deliberations among experts regarding the precise diagnosis of hyperprolactinemia. The event highlighted the importance for assay manufacturers to improve antibody specificity, reduce cross-reactivity, and use the PEG precipitation method to verify the true levels of monomeric prolactin. Creating a multidisciplinary collaboration framework, incorporating macroprolactin screening into clinical pathways, and advancing evidence-based revisions of international guidelines are essential for achieving precise diagnoses and optimizing the allocation of medical resources.
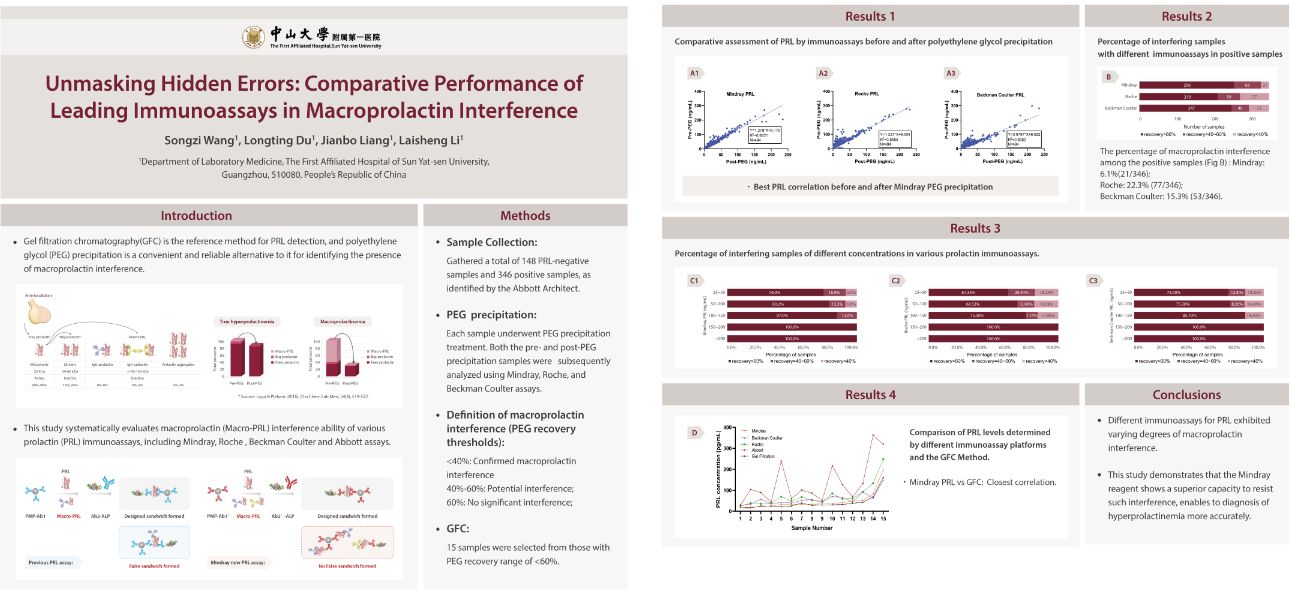
As medical technology continues to advance, we are confident that the precise diagnosis of hyperprolactinemia will soon be within reach. The contributions of innovative companies such as Mindray are paving the way towards achieving this goal. The PRL Workshop at this year's IFCC EuroMedLab congress has clearly injected new momentum and optimism into this endeavor.
