Ultrasound Journal 37 - Evaluation of liver graft by contrasted ultrasound and variables that may alter normality
2025-08-11
Special thanks to Jesús Alejandro, Sánchez Ramírez and Verónica Espinosa Cruz, INCMNSZ, MEXICO
Currently, liver transplantation is the first line of treatment for patients with acute and chronic end-stage liver disease. The number of transplants of all organs has grown exponentially year after year, in 2023 alone in Mexico, 297 liver graft transplants were performed, of which 52 were carried out at our institution [1].
Post-operative orthotopic liver transplant patients are followed closely after surgery to evaluate rejection and other complications. These follow-ups can be as early as 2h-24hrs after the procedure to evaluate patency of vascular structures and general perfusion of the organ, as well as to evaluate the upper abdomen for bruises, collections, etc.
The method of choice currently is Doppler ultrasound [2], which provides information on the parenchyma, the bile duct and vascular permeability, in addition to helping in the detection of complications such as biliary stenosis, hepatic artery stenosis and rejection, however, its effectiveness may be limited by several variables. In this context, contrast-enhanced ultrasound (CEUS) has proven to be a superior tool. This method significantly improves the evaluation of the liver graft by providing a more sensitive visualization of the flow in the hepatic artery and portal vein, this allows early detection of vascular complications, identification and location of biliary leaks and stenosis, evaluation and analysis of parenchymal perfusion [3]. However, its use still faces challenges, such as the lack of standardization of normal parameters in liver grafts.
The availability of ultrasonographic contrast varies by country. In Mexico, the most widely used and approved contrast for clinical use is SonoVue, manufactured by Bracco Suisse SA. At our institution, we have Mindray's Resona R9 equipment, which offers advanced functions such as the "Chrono-Parametric Mode CEUS" (CCPM), this mode allows the analysis of the perfusion of the organ through color mapping, providing information on the time it takes for the contrast to reach different regions of the evaluated organ [4].
With these technologies, post-transplant evaluation is more accurate, contributing to improved outcomes in transplant patients.
Liver CEUS has three overlapping vascular phases after contrast injection due to the double blood supply: the hepatic artery and the portal vein, which provide 25-30% and 70-75% respectively in non-cirrhotic conditions [5].
- Arterial phase begins at 10-20 seconds and ends at 30-45 seconds.
- Portal-venous phase begins at 30-45 seconds and ends at 120 seconds.
- Late phase begins after 120 seconds until the bubbles disappear (approximately 4-8 min).
These three phases are considered normal in a non-cirrhotic patient and not in a post-surgical state.
Therefore, the objective of this document is to show the importance of standardizing normal parameters of vascular phases and reducing the evaluation time in liver transplantation, as well as to identify variables that lead us to an ultrasonographic study with contrast with pathological findings.
Case presentation:
A 54-year-old patient with a diagnosis of liver cirrhosis secondary to autoimmune hepatitis, after orthotopic liver transplantation, who was presented to radiology for immediate post-surgical follow-up, important information was obtained such as organ preservation times (cold and warm ischemia time) as well as surgical technique, as additional data it was mentioned that the graft was of a greater volume expected for the patient's complexion. Grayscale, Doppler, and contrasted ultrasound were performed as postoperative follow-up at the time of the study, hemodynamically unstable with vasopressor support.
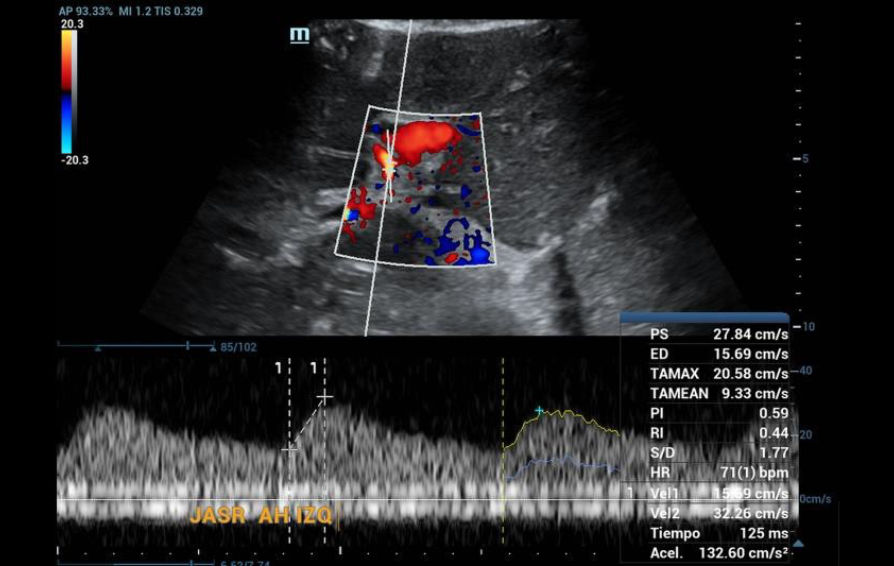
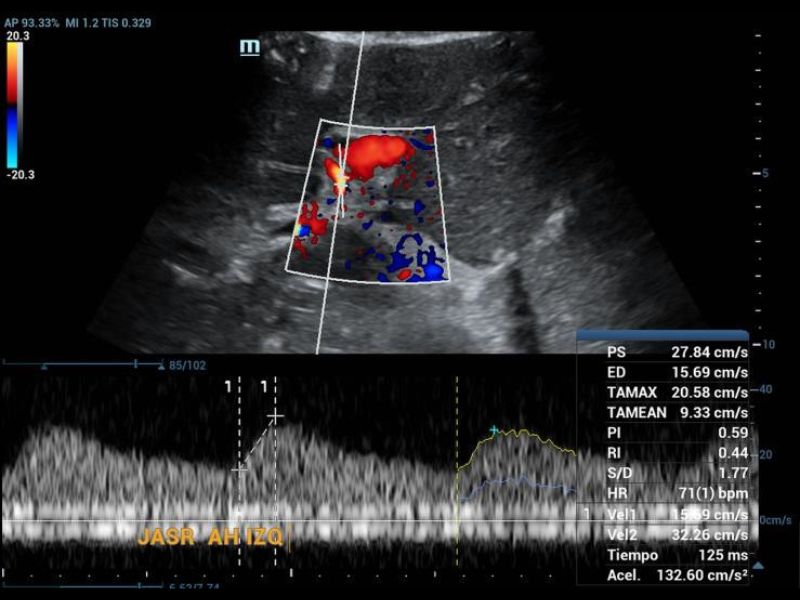
On examination of the grayscale graft, no findings of greater relevance were found in the parenchyma, but not when the vascular structures were evaluated in Doppler mode, since it was not possible to identify the right hepatic artery and the left was found with the morphology of tardus parvus (Figure 1), so the evaluation was directed towards a possible area of proximal stenosis, however, it wasn’t located.
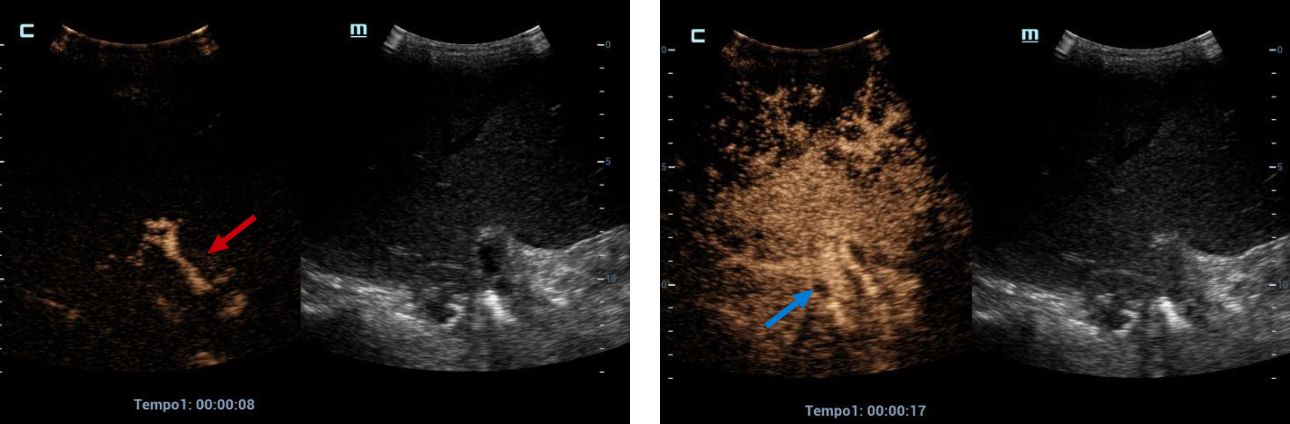
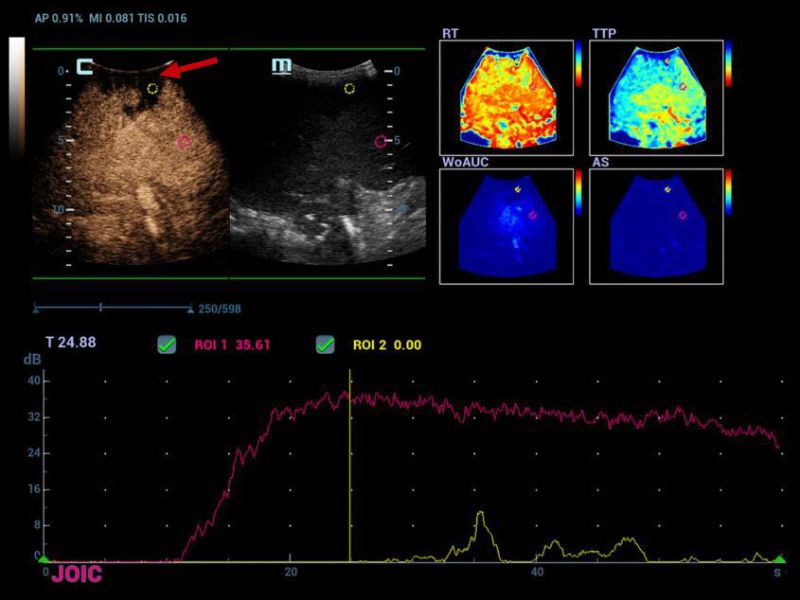
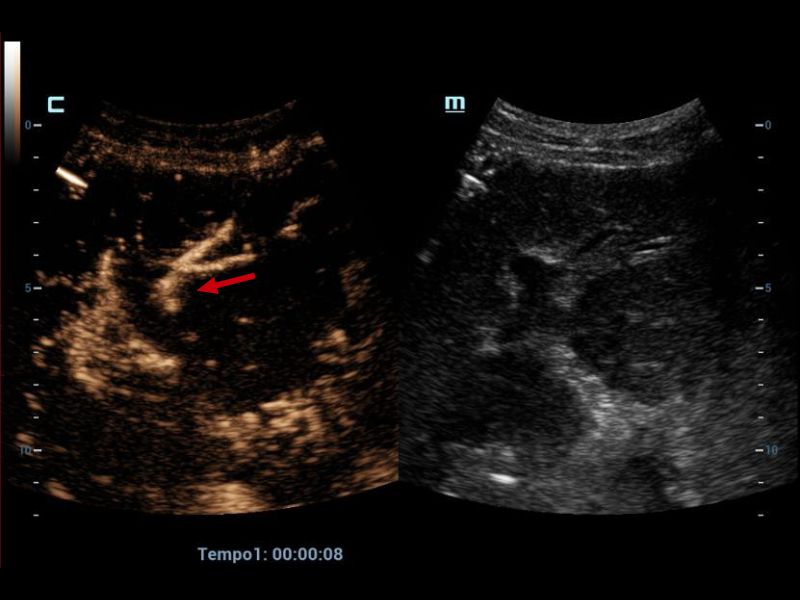
Therefore, it was decided to continue the assessment with contrasted ultrasound, an enhancement of vascular structures (Figures 2 and 3) can be observed about 8 seconds from the hepatic artery and 17 seconds from the portal vein without finding filling defects or areas of stenosis, however, in the portal phase of hepatic enhancement an area of hypoperfusion in segments 7/8, evidenced both by the live image and in the post-process of the perfusion curves and maps, this area was triangular morphology and subcapsular location (Figure 4), as well as the right hepatic artery is not visualized by this method, and the left was located at the level of the portal "H"(Figure 5).
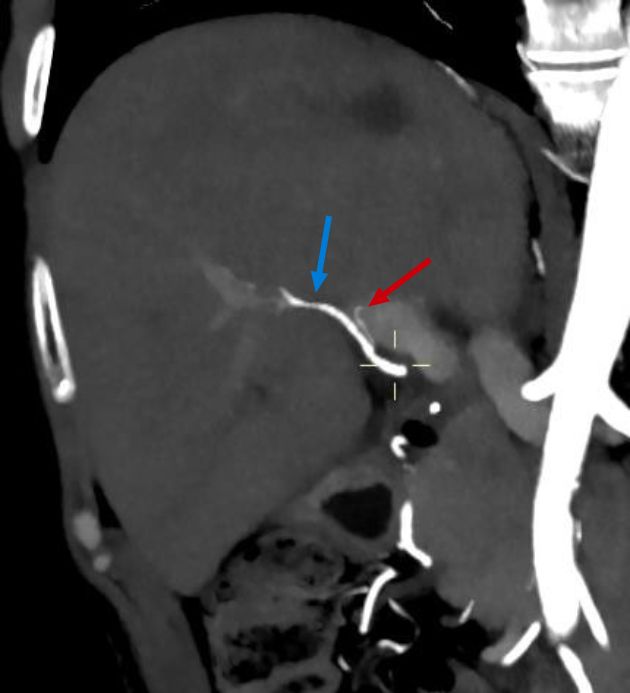
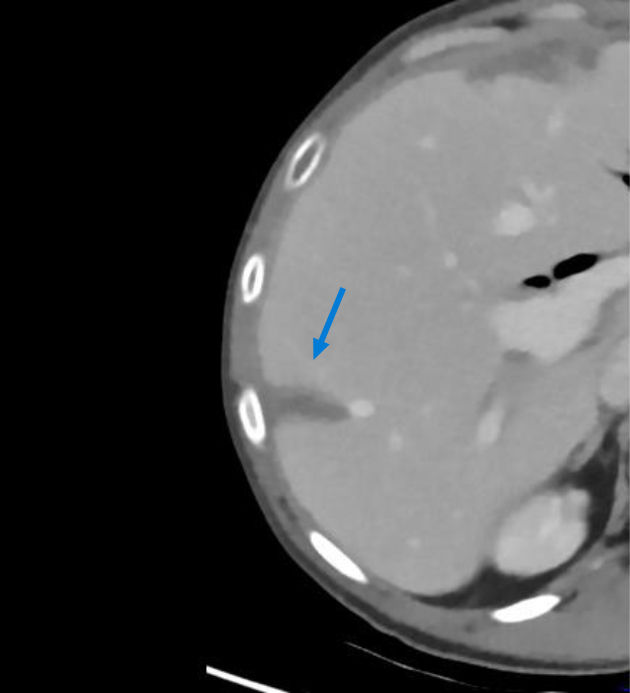
The CT angiography performed one month later showed a patent right hepatic artery and a left filiform hepatic artery (Figure 6), as well as an infarction area in segment 7/8 (Figure 7).
Discussion:
This case highlights how CEUS complements Doppler ultrasound in situations where technical limitations make vascular evaluation difficult. Early identification of areas of hypoperfusion or complications such as arterial stenosis or segmental infarction allows for better planning of postoperative management. In addition, it emphasizes the need to standardize normal parameters in the use of CEUS for transplant patients, with the aim of reducing interpretative variations and optimizing diagnosis times.
Reference:
[1]. Centro Nacional de Trasplantes (CENATRA) Estado Actual de Receptores, Donación y Trasplantes en México Anual 2023.
[2]. Chau, S. S., Beutler, B. D., Grant, E. G., & Tchelepi, H. (2024). Ultrasound innovations in abdominal radiology: multiparametic imaging in liver transplantation. Abdominal Radiology.
[3]. Lu, Q., Zhong, X. F., Huang, Z. X., Yu, B. Y., Yun, B., MA, Ling, W. W., Wu, H., Yang, J. Y., & Luo, Y.
(2011). Role of contrast-enhanced ultrasound in decision support for diagnosis and treatment of hepatic artery thrombosis after liver transplantation. European Journal Of Radiology, 81(3), e338-e343.
[4]. Jung, E., Dong, Y., & Jung, F. (2023). Current aspects of multimodal ultrasound liver diagnostics using contrast-enhanced ultrasonography (CEUS), fat evaluation, fibrosis assessment, and perfusion analysis – An update. Clinical Hemorheology And Microcirculation, 83(2), 181-193.
[5]. Dietrich, C. F., Nolsøe, C. P., Barr, R. G., Berzigotti, A., Burns, P. N., Cantisani, V., Chammas, M. C., Chaubal, N., Choi, B. I., Clevert, D., Cui, X., Dong, Y., D’Onofrio, M., Fowlkes, J. B., Gilja, O. H., Huang, P., Ignee, A., Jenssen, C., Kono, Y.Zheng, R. (2020). Guidelines and Good Clinical Practice Recommendations for Contrast-Enhanced Ultrasound (CEUS) in the Liver–Update 2020 WFUMB in Cooperation with EFSUMB, AFSUMB, AIUM, and FLAUS. Biology, 46(10), 2579-2604.