Besides special scenarios such as prosthetic joint infection and febrile neutropenia, sCD14-ST can also be used in commonly seen clinical scenarios such as neonatal and adult sepsis and early infection in trauma.

Sepsis is a life threatening illness caused by your body’s response to an infection [1]. When dealing with sepsis, early recognition and treatment matters [2].
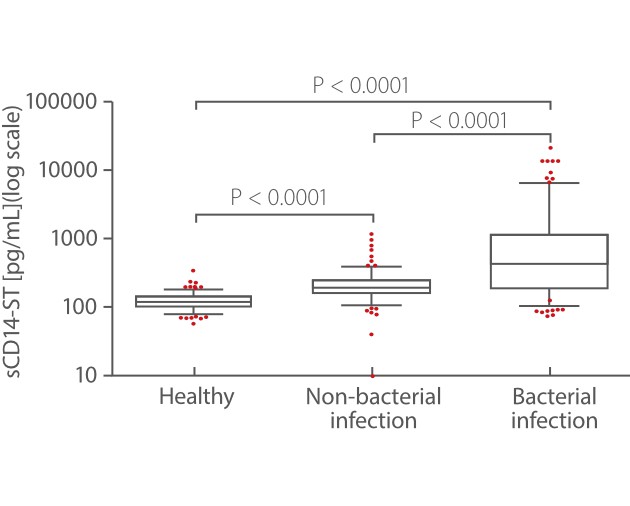
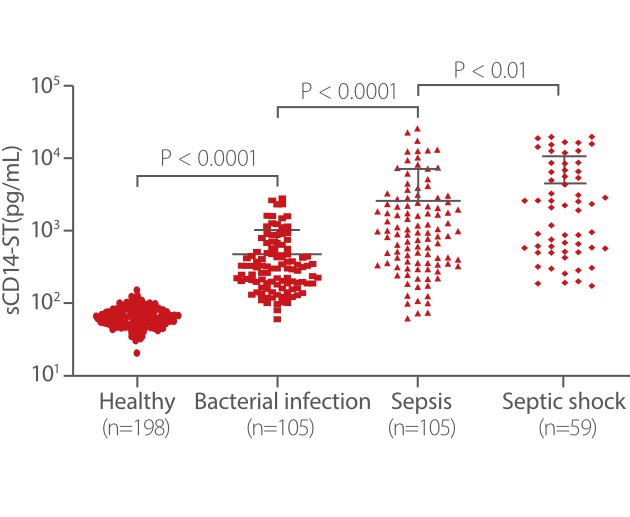
From Mindray’s internal and external studies, sCD14-ST is found to be an accurate biomarker for bacterial infection. Mindray sCD14-ST assay exhibits significantly higher concentration levels in the bacterial infection disease group than in nonbacterial infectious disease and healthy groups [3].
Neonatal sepsis is a blood infection that occurs in an infant younger than 90 days old [4]. Early diagnosis and prompt treatment of neonatal sepsis can be challenging as its signs and symptoms are nonspecific. Although a number of studies have been conducted on various diagnostic markers, further research is required to identify a biomarker with high diagnostic accuracy and validity [5].
From some studies, sCD14-ST is found to be a valuable biomarker for neonate sepsis, playing an important role in diagnosis and evaluation of severity. Its levels are significantly higher in neonatal sepsis than in non-infective systemic inflammatory response syndrome (SIRS) and normal control groups, suggesting that it could be an indicator for identifying neonatal sepsis, SIRS and normal control group [6].

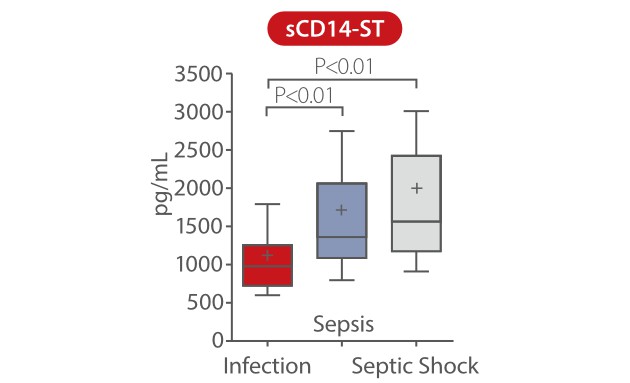
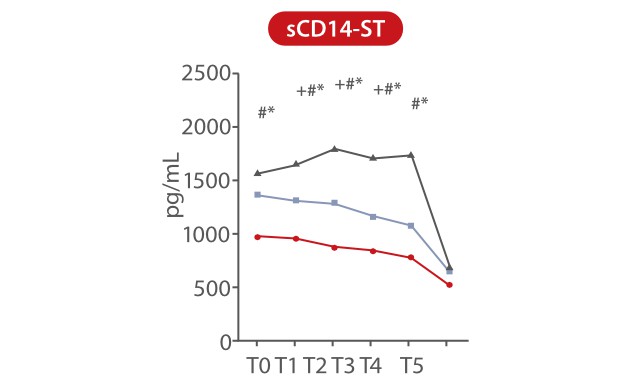
In a study published by Pietrasanta, data showed that sCD14-ST is an accurate biomarker for early identification of septic neonates at a higher risk of rapid derangement of clinical conditions, which are preferably dealt with by a tailored medical and therapeutic approach. sCD14-ST levels at T0 were significantly higher in neonates with sepsis and septic shock than in those with infection. During the first 48h from the onset of symptoms, sCD14-ST progressively increased in neonates with septic shock, while it remained stable or decreased in neonates with sepsis or infection [7].
P<0.01 marked as follow: *, infection vs. sepsis; #, infection vs. septic shock; +, sepsis vs. septic shock
T0: onset of symptoms. T1: 12h, T2: 24h, T3: 36h, T4: 48h, T5: end of antibiotic therapy
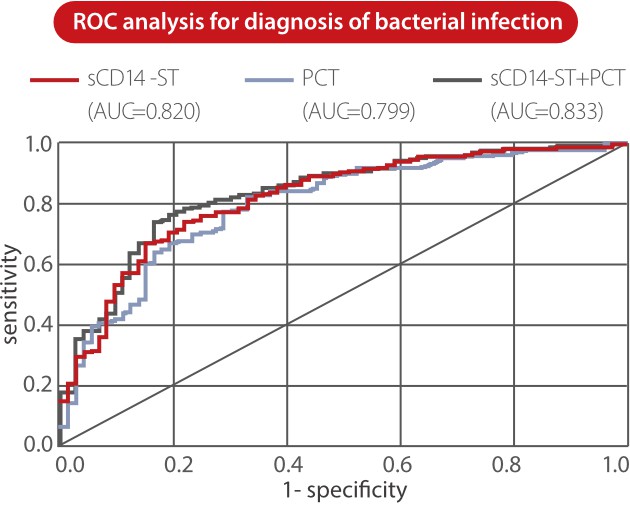
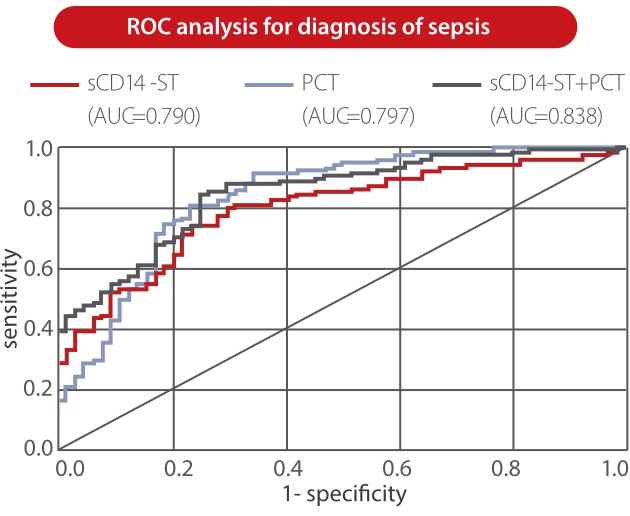
From Mindray clinical validation studies, sCD14-ST is proven to be a predictive marker for adult sepsis. The level of Mindray sCD14-ST is significantly higher in sepsis and septic shock groups than in bacterial infection and healthy groups. The ROC analysis showed that the sCD14-ST biomarker has good diagnosis performance in distinguishing between ICU patients with sepsis and those without sepsis [3].
Also, sCD14-ST is a sensitive biomarker for the assessment of sepsis severity in ICU patients. In this study, the sCD14-ST levels were significantly different between severely ill (APACHE II ≤ 15) and critically ill (APACHE II >15) groups, and the PCT and IL-6 levels showed no statistically significant difference between these two groups [3].

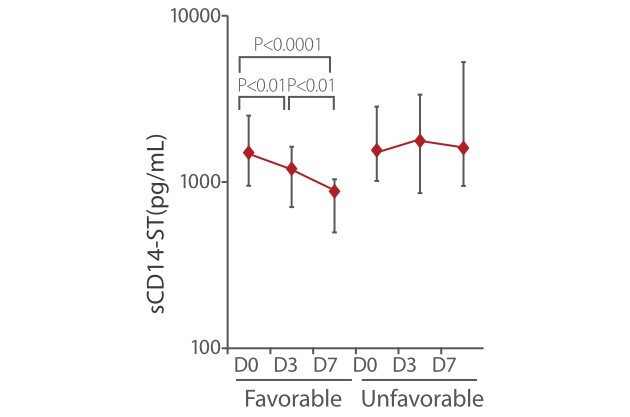
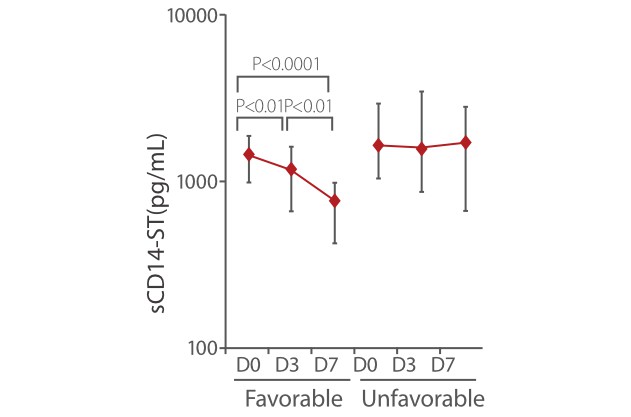
Other studies also showed that sCD14-ST is a powerful monitoring tool for the treatment of sepsis. In the SOFA and APACHE II favorable group, the sCD14-ST levels on D3 and D7 were significantly lower than levels measured at the time of admission. Meanwhile, in the SOFA and APACHE II unfavorable group, the sCD14-ST levels on D7 were not significantly lower than the levels measured at the time of admission [8].
Sepsis is a leading cause of in-hospital death. The incidence of sepsis due to multidrug resistant organisms (MDROs) is rapidly increasing. MDROs can colonize trauma patients who survive to initial injury can develop hospital-acquired infections. Without prompt diagnosis and early treatment these infections can lead to sepsis with very poor prognosis [9].
From relevant studies, sCD14-ST is proven to be a superior biomarker for early differentiation of infection in trauma patients. Plasma sCD14-ST levels within the first 3 days of admission were only significantly increased in the infected trauma group, but not in the noninfected trauma and sterile group. sCD14-ST is also specified in the presence of infection in trauma patients [10].

Although sCD14-ST is a novel biomarker, many studies have revealed that it can be used in various clinical scenarios while offering high sensitivity and specificity. sCD14-ST overcomes the drawbacks of traditional biomarkers to help solve pain points in clinical practice.

If you have interest in this inflammation biomarker, please register to watch Mindray IPS (IL-6, PCT and sCD14-ST) online launch event on 1st December, 2022:
https://www.mindray.com/en/events/webinar-on-il-6-scd14-st
References
[1] https://www.healthline.com/health/sepsis
[2] https://www.osfhealthcare.org/blog/sepsis-timematters/
[3] Mindray clinical validation
[4] https://medlineplus.gov/ency/article/007303.htm
[5] Birju A Shah and James F Padbury, Neonatal sepsis - An old problem with new insights. Virulence. 2014 Jan 1; 5(1): 170–178. Published online 2013 Nov 1. doi: 10.4161/viru.26906
[6] Chen L, et al. Soluble CD14 subtype (sCD14-ST) is a biomarker for neonatal sepsis. Int J Clin Exp Pathol. 2017;10(9):9718-9724.
[7] Pietrasanta, C.et al. Presepsin (Soluble CD14 Subtype) as an Early Marker of Neonatal Sepsis and Septic Shock: A Prospective Diagnostic Trial. Antibiotics 2021, 10, 580
[8] Endo S, et al., Presepsin as a powerful monitoring tool for the prognosis and treatment of sepsis: A multicenter prospective study, J Infect Chemother (2013)
[9] https://infectionsinsurgery.org/infections-in-traumapatients/
[10] Kang, Jian*,†; etal. Early Differential Value of Plasma Presepsin on Infection of Trauma Patients, SHOCK: September 2019 - Volume 52 - Issue 3 - p 362-369