Mindray Multi-center Joint Study on Reference Intervals of Lung Cancer Biomarkers in China
2022-11-01
Study Background
Cancer is a leading cause of death worldwide, accounting for nearly 10 million deaths in 2020 [1]. Lung cancer (both small cell and non-small cell) is the second most common among all cancer types (excluding skin cancer), accounting for 2.21 million new cases and 1.80 million deaths in 2020 [1].
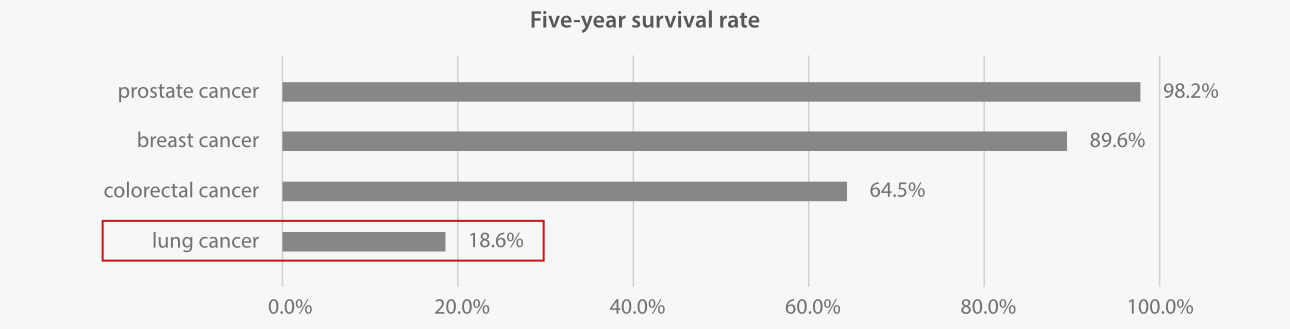
Doctors use lung cancer survival rates or survival statistics to tell you the percentage of people who survive a certain type and stage of cancer for a specific amount of time[3]. Lung cancer has a lower 5-year survival rate than many other leading cancer types, which is 18.6% only. More than half of people with lung cancer die within one year of being diagnosed [4].
Therefore, improving the early diagnosis of lung cancer is critical for the timely treatment and prognosis monitoring of lung cancer patients. That’s where tumor markers come in.
A tumor marker is produced by cancer cells or other cells of the body in response to cancers or certain benign (noncancerous) conditions [4]. The presence or absence of these markers can indicate the nature of the tumor and guide the diagnosis, classification, prognosis and treatment of tumors.
However, the reference intervals of tumor markers are not fully applicable for specific populations such as Chinese patients, and the sensitivity and specificity of a single marker are still far from meeting the clinical needs.
Mindray Study
Patients and methods
To better provide the reference intervals of lung cancer biomarkers applicable for Chinese patients, Mindray worked with a number of well-known hospitals across the country to launch the “Multi-center Joint Study on Reference Intervals of Lung Cancer Biomarkers in Chinese People” project in October 2018.
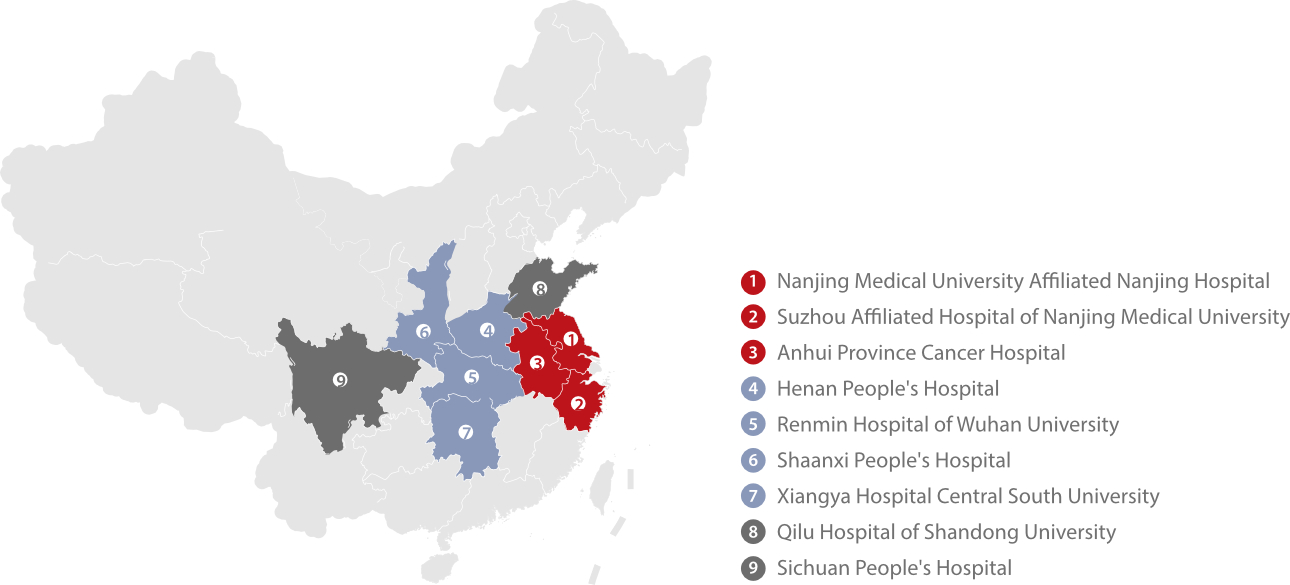
With the aim to improve the accuracy of lung cancer diagnosis and facilitate the early diagnosis of lung cancer, the study was divided into two parts: evaluating the clinical value of lung cancer markers and establishing a risk prediction model for early screening of lung cancer. For the first part, the research team took NSE, CEA, CA125, SCC, CYFRA21-1 and ProGRP as the research objects and utilized big data analysis to identify reference intervals applicable for Chinese people. For the other part, they explored a set of serum tumor marker combinations with high sensitivity and high specificity for lung cancer screening and established a Nomogram prediction model based on serum tumor markers.
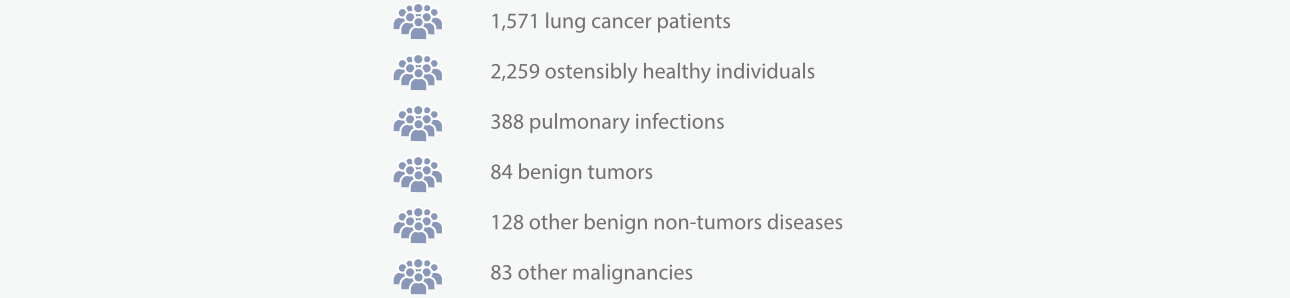
The study enrolled 1,571 lung cancer patients (1,056 males and 515 females, aged 21–90), 2,259 ostensibly healthy individuals (990 males and 1,269 females, aged 13–87), 388 patients with pulmonary infections (228 males and 160 females, aged 15–92), 84 with benign tumors (19 males and 65 females, aged 22–79), 128 with other benign non-tumors diseases (75 males and 53 females, aged 14–92), and 83 with other malignancies (45 males and 38 females, aged 22–90) [5-6].
Results
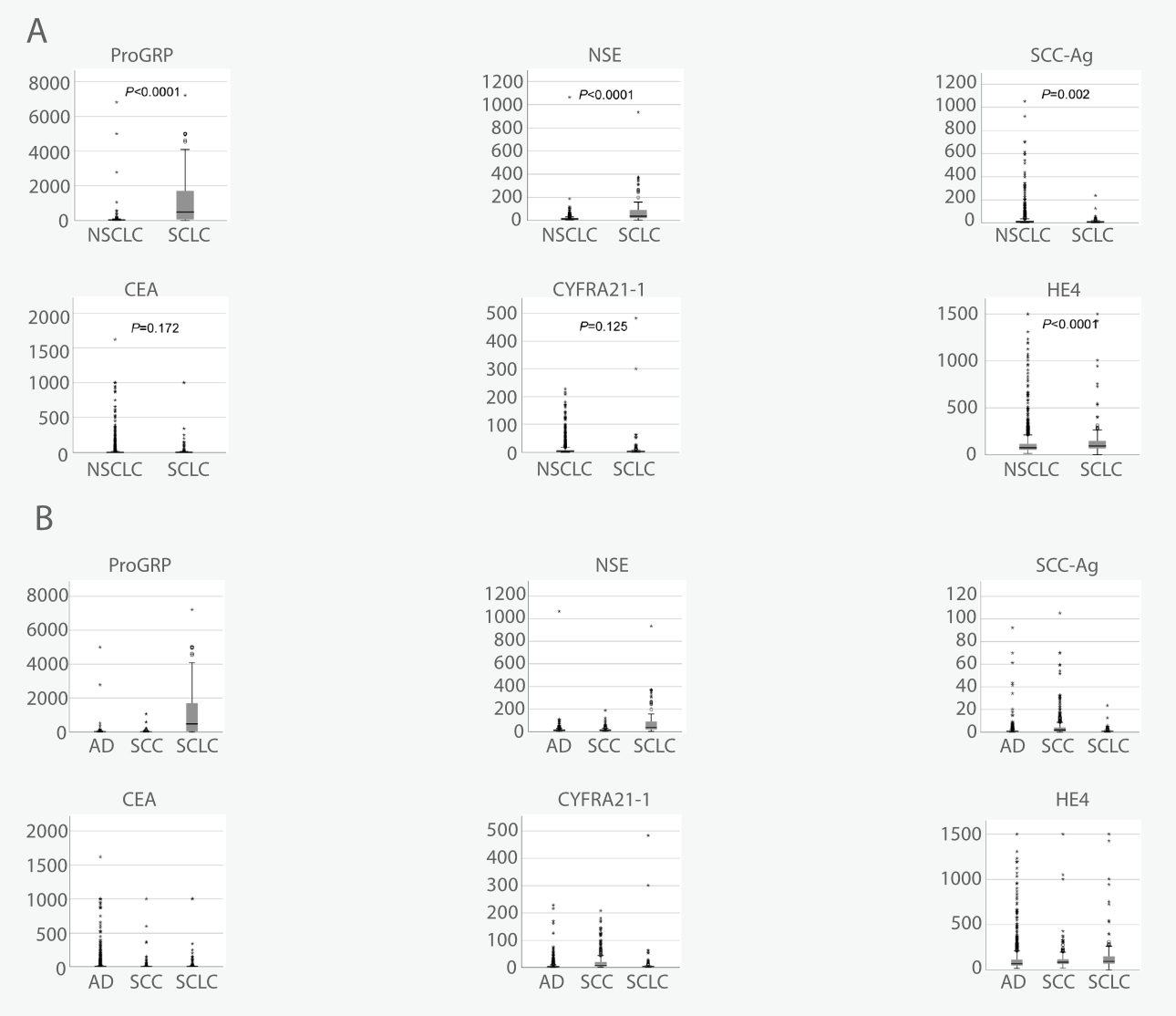
The study revealed that serum ProGRP, NSE, SCC-Ag, CEA, CYFRA21-1, and HE4 can be used to characterize lung cancer from other diseases and healthy individuals [5-6].

NSE and ProGRP are two ideal biomarkers for characterizing SCLC, while SCC-Ag is a fair marker for NSCLC, specifically for SCC diagnosis. Elevation of serum CEA or CYFRA21-1 is indicative of the SCLC or NSCLC histotype. HE4 exhibits high specificity to SCLC, and it can offer enhanced diagnostic sensitivity when used in combination with other biomarkers. Thus, HE4 can be a potential serum biomarker for lung cancer diagnosis. New biomarkers with high sensitivity to adenocarcinoma of the lung are needed [5-6].
The above-mentioned research has been published in the Journal of Clinical Laboratory Analysis.
In 2020, the TBM-Mindray Model was established based on gender, age and lung cancer tumor markers, forming a lung cancer risk prediction model applicable for Chinese people. This model shows good performance with high sensitivity (89%) and specificity (86%).
TBM: Tumor Bio-Marker, a college of the China Anti-Cancer Association
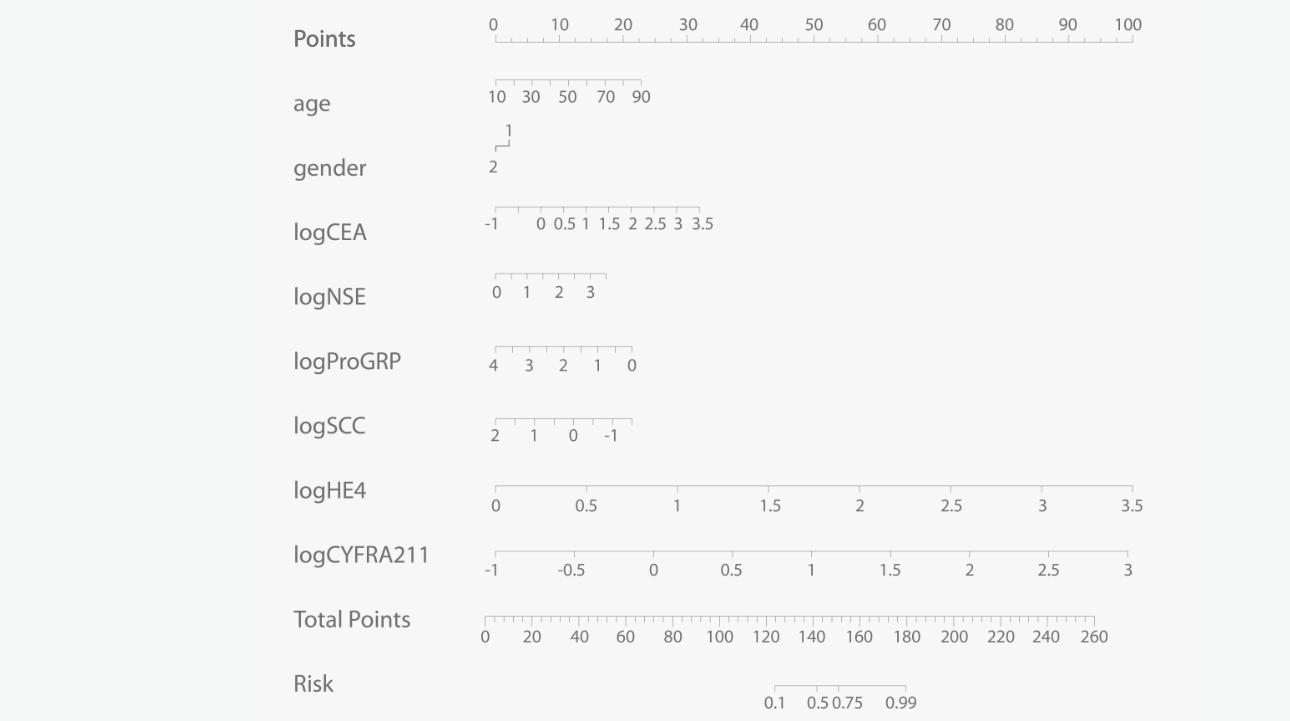
The TBM-Mindray Model delivers excellent performance and the calculation parameters it requires can be obtained easily, making it a widely applicable testing technology to support the screening of lung cancer in primary healthcare. Mindray is looking forward to collaborating with more hospitals and laboratories around the world on regional multicenter research on lung cancer and other diseases, and establishing more expert consensus for improving the quality of clinical testing.

Reference:
[1] https://www.who.int/news-room/fact-sheets/detail/cancer
[2] https://www.cancer.org/cancer/lung-cancer/about/key-statistics.html
[3] https://www.lung.org/lung-health-diseases/lung-disease-lookup/lung-cancer/treatment/your-lung-cancer-your-goals
[4] U.S. National Institute Of Health, National Cancer Institute. SEER Cancer Statistics Review, 1975–2015.
[5] New insights into the diagnostic characteristics and clinical application of serum biomarkers for lung cancer, and human epididymis protein 4 as a new biomarker? doi:10.4149/neo_2022_xxx
[6] Li M, Zhang Y, Jiang L et al. New insights into the diagnostic characteristics and clinical application of serum biomarkers for lung cancer, and human epididymis protein 4 as a new biomarker? Neoplasma. 2022 Apr 26:220207N144.