Unlocking Microbiology: FA-N Series Offers Blood Volume Measurement to Enhance Lab Quality Management
2025-07-03

Studies have shown that blood culture specimens with collection volumes of 8-10 mL demonstrate faster time-to-positivity (5-20 hours faster on average, p<0.01) [1], along with significantly improved positive detection rates (10% increase in proportion, 3% higher detection rate, p<0.01) [2]. Therefore, blood volume monitoring has garnered considerable clinical attention and holds significant importance.
To address clinical pain points in traditional microbial blood volume detection methods, Mindray has launched the FA-N Series Fully Automated Microbial Culture System. Featuring innovative design, this system provides automated comprehensive blood volume detection starting from a 120-cavity configuration. Combining high-precision measurement, user-friendly operation, and robust clinical support functions, it fully complies with microbial industry standards and ISO15189 quality management requirements, delivering unprecedented technological breakthroughs in blood culture collection volume management.
Blood volume is critical
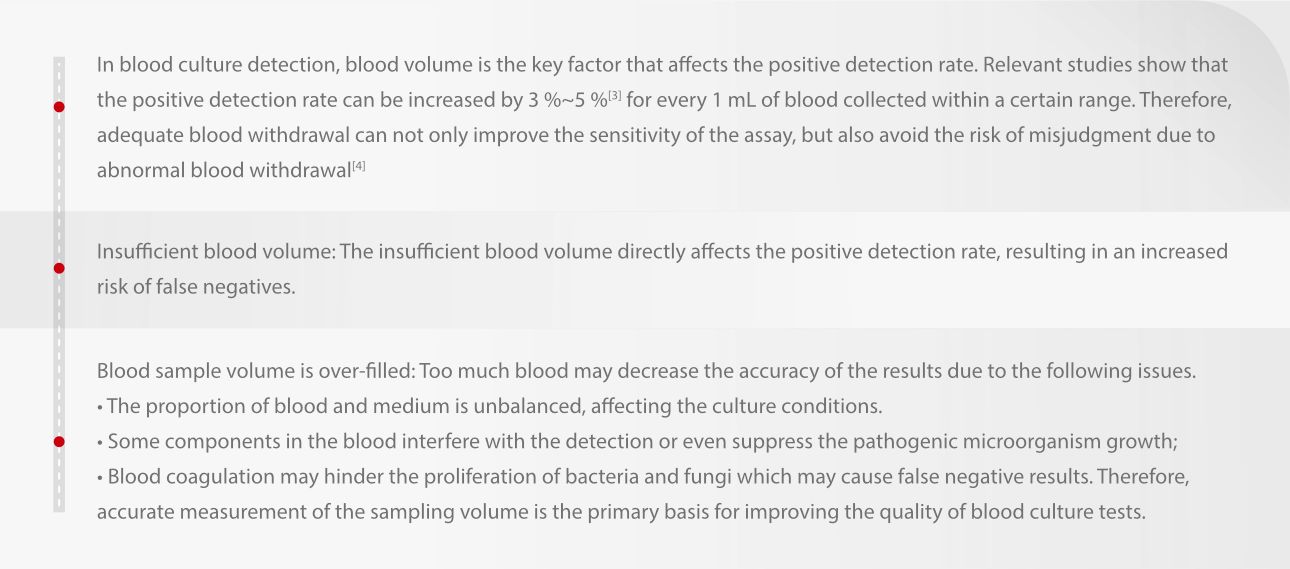
Laboratory management and industry standards have clear requirements

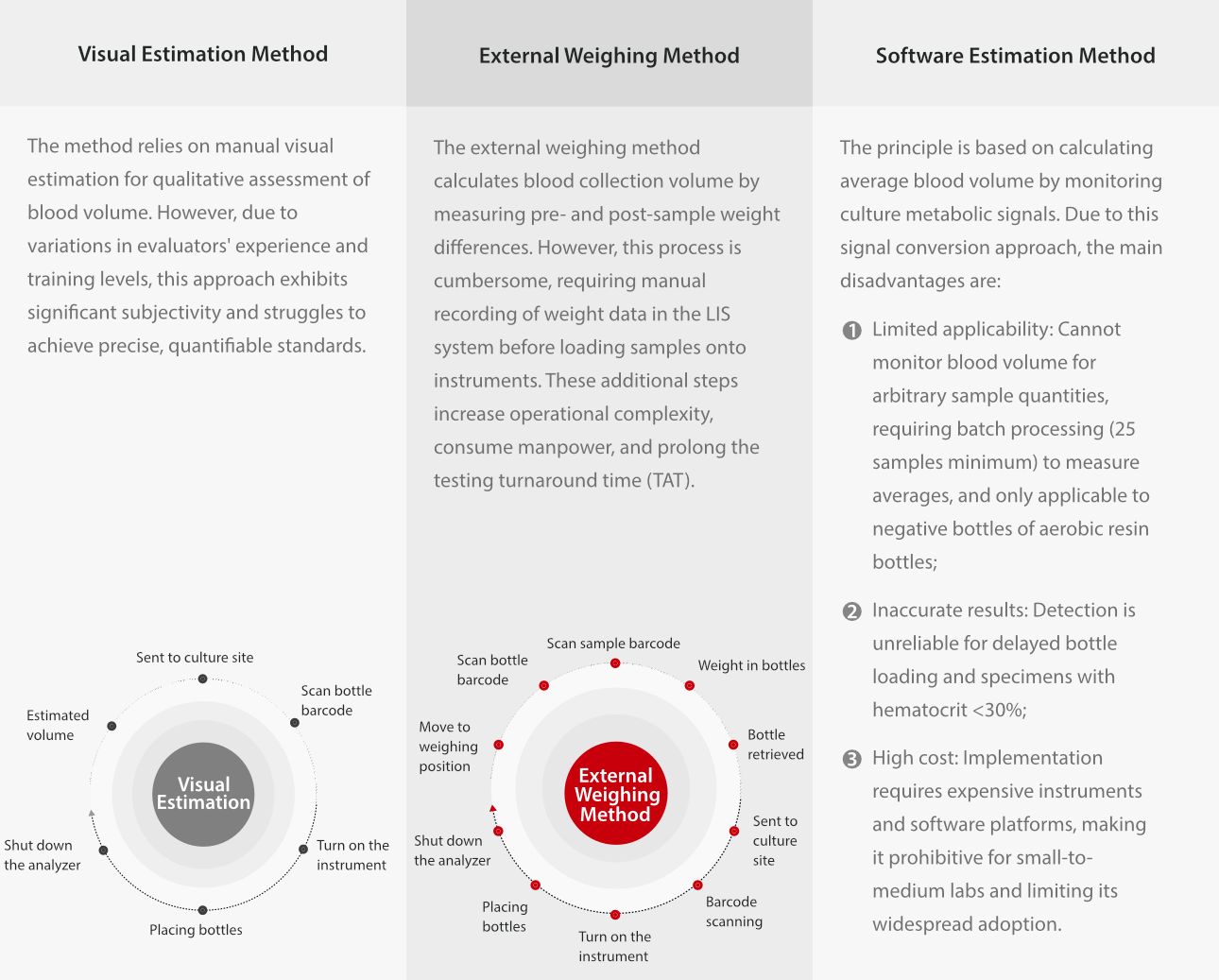
Current shortcomings in blood collection volume detection and methodologies
Currently, laboratories mainly use the following three common methods to evaluate blood volume, but all have certain limitations:
Mindray's comprehensive blood volume monitoring solution
Precise, Intelligent, Standardized – Elevate Specimen Blood Volume Quality Management in One Step. The Preferred Equipment for Laboratory Blood Volume Management.
Mindray FA-N Series Comprehensive Blood Volume Monitoring System integrates scientific rigor, automation, and digitalization, bringing revolutionary innovation to the field of blood culture volume detection.
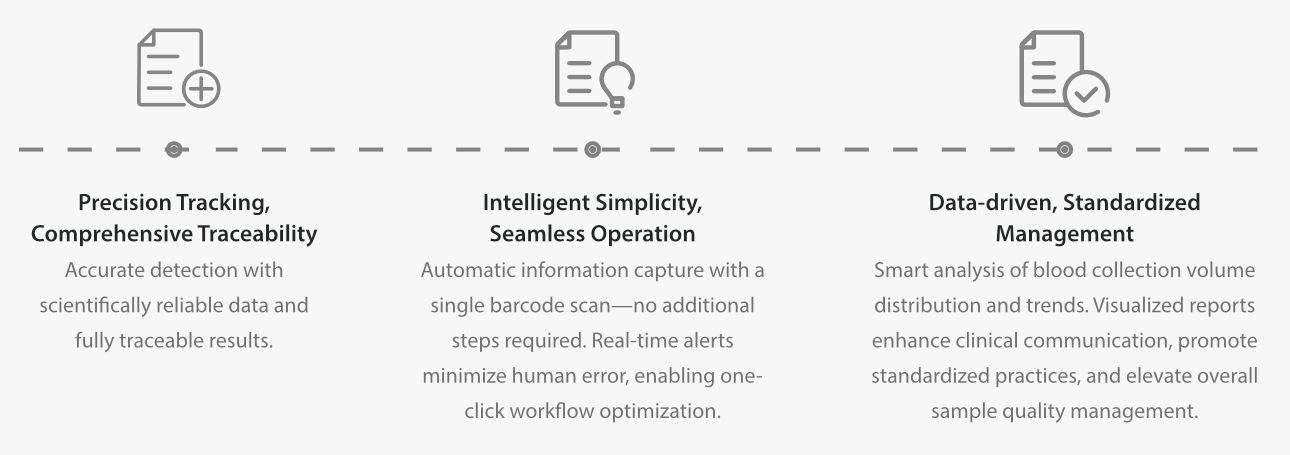
The Mindray FA-N Series Automated Microbial Culture System, with its innovative blood volume detection function, powerful statistical analysis capabilities, and user-friendly operation experience, not only fully complies with CAP, ISO15189, and industry standards but also meets the needs of traceability management and quality control report generation by completely preserving historical blood volume data. Additionally, it significantly improves the positive detection rate of blood cultures, reduces the incidence of false negatives and false positives, provides high-quality microbiological diagnostic support for standardized laboratory management and clinical practice, and drives a qualitative leap in blood culture testing.
References
[1] Jin Jianwen, Jia Lei, et al. Discussion on the Relationship between Positive Reporting Time of Blood Culture and Sample Blood Volume. Chinese Journal of Sanitary Inspection, 2021,31(17):2112-2114
[2] Li Aqiong, Lin Jiayi, Chen Xiaoting. Research on the Relationship between the Positive Rate of Blood Culture and the Blood volume collected by nurses for blood culture. Heilongjiang Medicine and Pharmacy, 2025.48(3):7-10.
[3] Bouza, Emilio, et al. Is the volume of blood cultured still a significant factor in the diagnosis of bloodstream infections . Journal of clinical microbiology 45.9 (2007): 2765-2769
[4] CLSI. Principles and Procedures for Blood Cultures; 2nd ed. CLSI guideline M47. Clinical and Laboratory Standards Institute; 2023.
