Due to the increased awareness of prevention and the improvement of diagnosis and treatment, HIV infection has become a manageable chronic health condition. Even so, as an illness having claimed about 33 million lives so far, HIV infection continues to be a major global public health issue.

Who Should Be Screened for the HIV Infection?
Identification and early treatment of HIV infection could be beneficial to reducing the risk of AIDS- related events or death. According to the recommendation of US Preventive Services Task Force, the following population should take the screening test: adolescents and adults aged 15 to 65 years, younger adolescents and older adults who are at increased risk of infection, and all pregnant women, including those who are in labor or at delivery with unknown HIV infection status.
However, the 3 types listed above are not the only ones who are at risk. Those who fit the follow descriptions should also consider taking HIV screening tests.
- High risk sexual behaviors

- Needle sharing among drug users

- Invasive procedures

- Needlestick injuries of healthcare workers

- HIV-positive moms giving birth or breastfeeding

As part of screening and diagnosis of HIV infections, laboratory testing plays a crucial role.
There are three types of HIV laboratory testing methods:

Antigen/antibody tests look for both HIV antibodies and antigens (e.g. p24 antigen). They are recommended for testing HIV in routine labs and are now commonly used globally.
The 4th generation HIV antigen/antibody test detects the HIV p24 antigen and both HIV-1/2 antibodies. This enables clinicians to identify HIV earlier than previous generations of tests. Even though POC HIV tests are relatively convenient, it is still recommended that testing for non-emergency cases should be performed using laboratory-based 4th generation immunoassays, given their established performance.
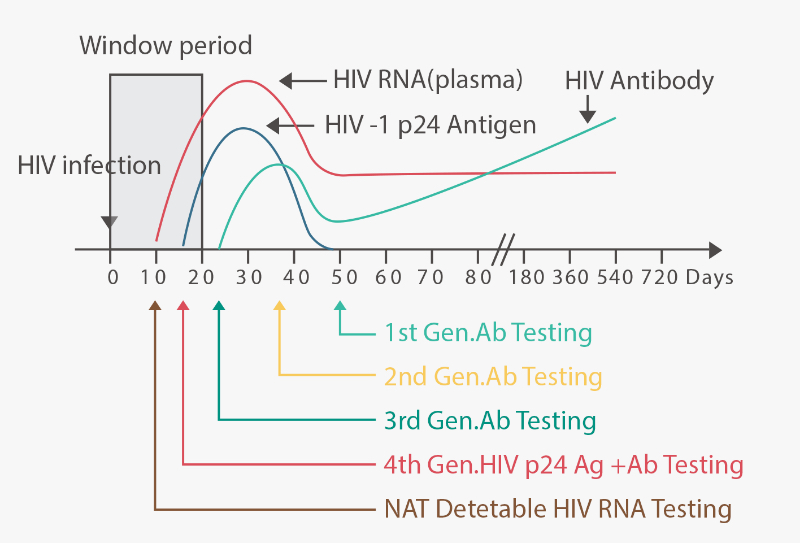
New CDC Recommendations for HIV Testing in Laboratories

Mindary 4th generation HIV testing
As the first Chinese medical device company to receive List A CE marking for infectious diseases tests, Mindray provides highly reliable HIV CLIA (Chemiluminescence Immunoassay) test kits.
References:
1. WHO(World Health Organization) website [https://www.who.int/news-room/fact-sheets/detail/hiv-aids];
2. US Preventive Services Task Force. Screening for HIV Infection: US Preventive Services Task Force Recommendation Statement. JAMA. 2019;321(23):2326–2336. doi:10.1001/jama.2019.6587;
3. UK NHS(National Health Service) [https://www.nhs.uk/conditions/hiv-and-aids/causes/];
4. US CDC(US Centers for Disease Control and Prevention [https://www.cdc.gov/hiv/testing/index.html];
5. website,Best Practices for HIV-1/2 Screening: When to Test and What to Test





