HbA1c is the cornerstone of diabetes care. It is widely used as a treatment goal as well as to predict the risk of complications developing in diabetes mellitus patients.
There are many analytical methods used in measuring HbA1c. The heterogeneity of methodology which is generated concerns the comparability and usability of HbA1c. In order to obtain comparable results of HbA1c by different methods, the standardization of these methods is essential.
Commercial Methods of HbA1c Measurement
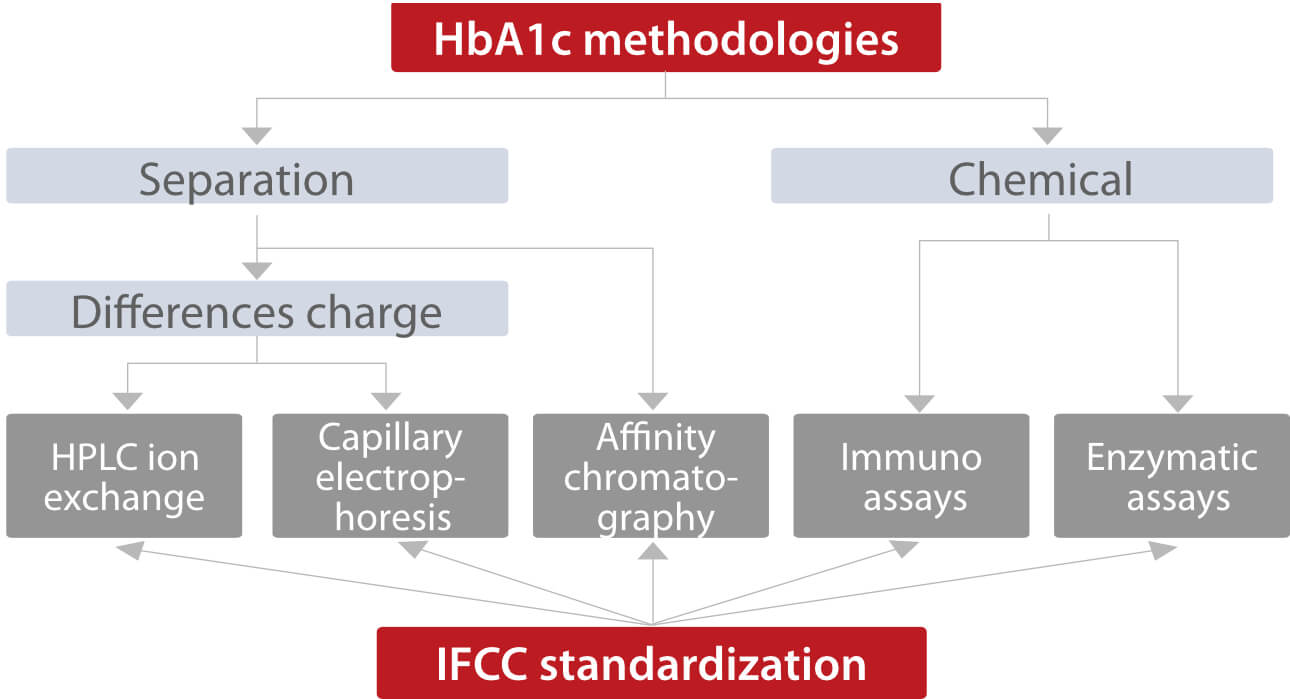
There are two major analytical concepts: one is based on the separation of Hb fractions, and the other is based on chemical reactions (Fig.1). Although different analytes are measured by these methods, assays can be standardized according to the Reference Measurement Procedure (RMP) of the International Federation of Clinical Chemistry (IFCC)[1].
Global Standardization of HbA1c Measurement
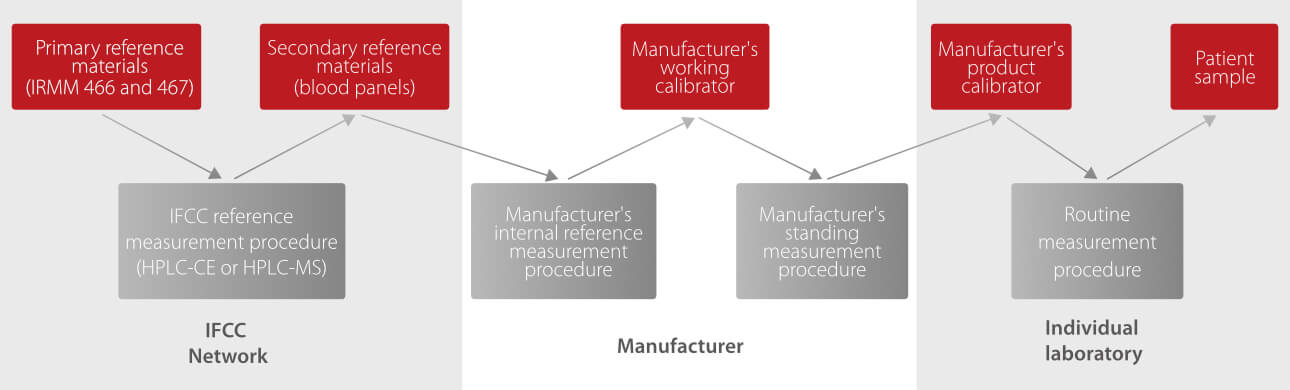
The International Federation of Clinical Chemistry and Laboratory Medicine (IFCC) Working Group on the Standardization of HbA1c has established an international reference measurement system for HbA1c. Glycated and non-glycated N-terminal hexapeptides are separated by reversed phase high-performance liquid chromatography (HPLC) followed by identification and quantification by capillary electrophoresis (CE) or electrospray ionization mass spectrometry (ESI-MS).
The successful preparation of pure HbA1c calibration material should lead to further improvements in inter-method and inter-laboratory variability.
In 2007, a meeting was held in Milan, and a consensus statement was published jointly by American Diabetes Association (ADA), European Association for the Study of Diabetes (EASD), IFCC and International Diabetes Federation (IDF)[2]. Their recommendations were:
“HbA1c test results should be standardized worldwide, including the reference system and results reporting. The new IFCC reference system for HbA1c represents the only valid anchor to implement standardization of the measurement. HbA1c results are to be reported worldwide in IFCC units (mmol/mol) and derived NGSP units (%), using the IFCC-NGSP master equation.”
Mindray’ s enzymatic HbA1c can be traceable to the IFCC reference standard, and has high accuracy with small bias and low CV%, helping doctors to provide better diabetes diagnosis and management.

Method Comparisons among Commercialized Methods
Mindray R&D compared its enzymatic HbA1c method with other available laboratory methods. The assay principles of these other methods, from Bio-Rad, Trinity Biotech and Roche, use iron-exchange chromatography, boronate-affinity chromatography and immunoassay respectively.
All methods can be traceable to the IFCC Reference Method.
Every sample was tested in duplicate. Each method was calibrated according to the manufacturer’ s instructions and two control materials, including normal and high levels, were tested as internal quality control. HbA1c results were reported as a percentage of the total hemoglobin. Correlation, regression and relative bias analysis are shown in Fig.4, Fig.5 and Fig.6.
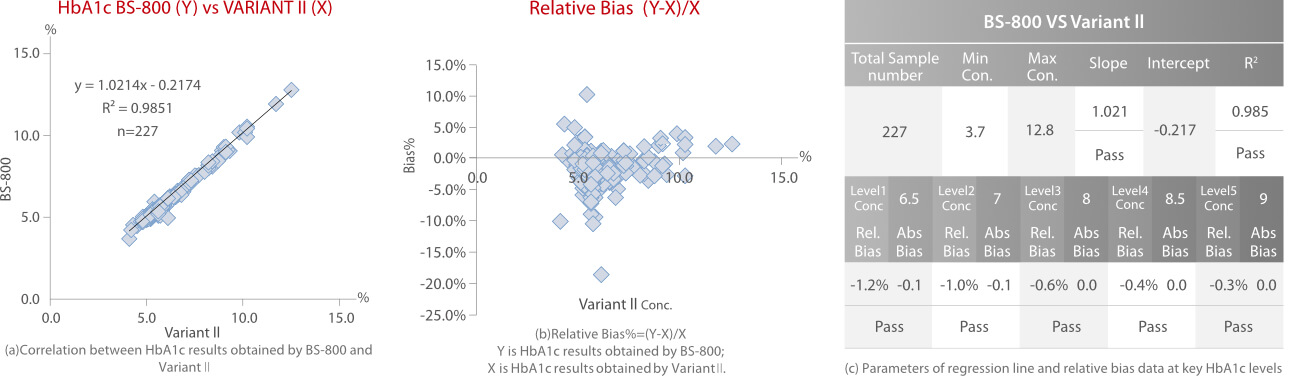
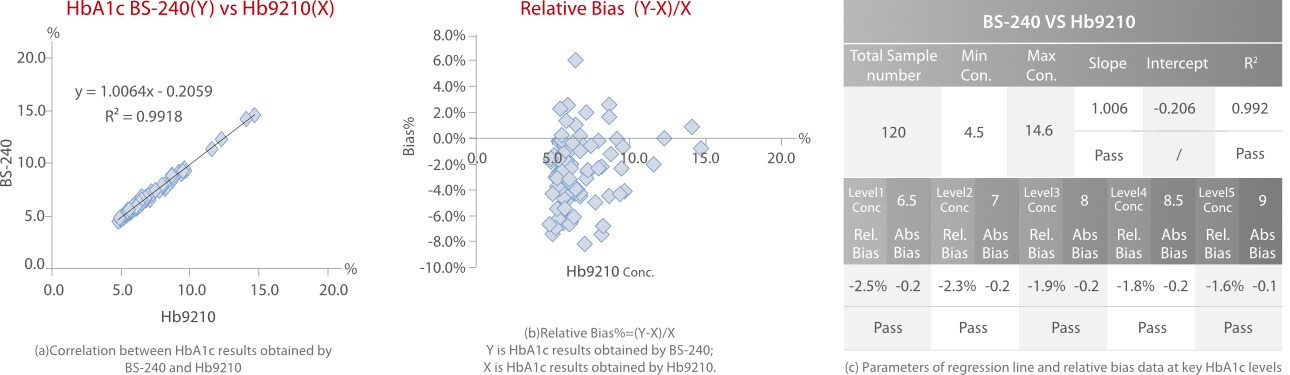
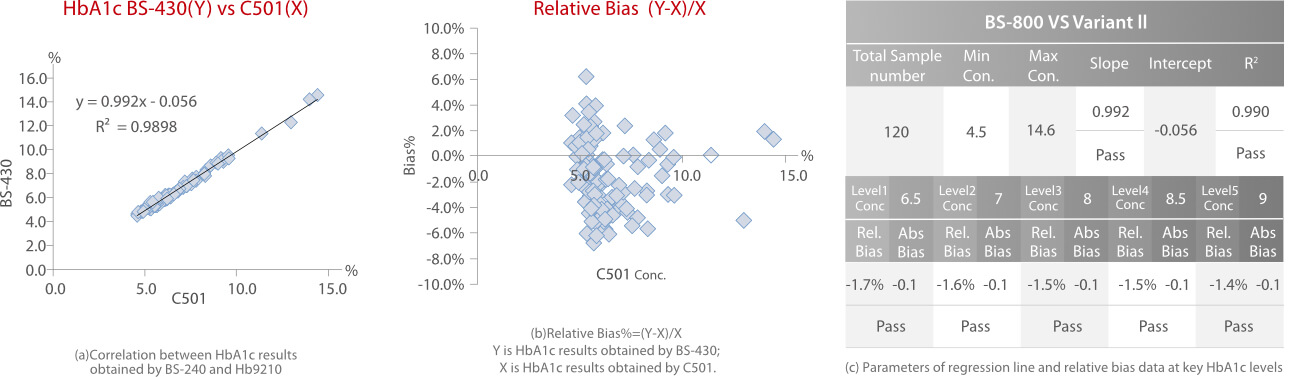
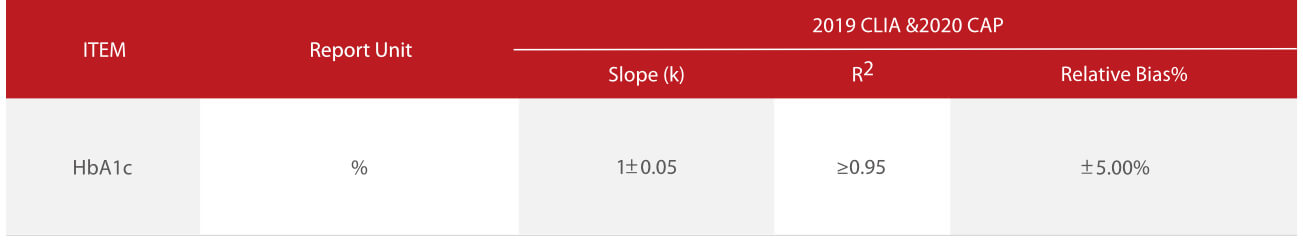
According to the HbA1c method comparison criteria from 2019 CLIA [3]and 2020 CAP [4], Mindray’ s enzymatic HbA1c method showed good correlations (R2>0.96) with Bio-Rad Variant II HPLC (ion-exchange chromatography), Trinity Hb9210 HPLC (boronate affinity) and Roche C501 (immunoturbidimetry). The relative bias% at key HbA1c levels between each of the two methods is less than 5%.
Conclusion
The global standardization of HbA1c measurement makes HbA1c results obtained from different methods more accurate and comparable.
Mindray enzymatic HbA1c is well in line with other commercialized testing methods. It has proven to be a reliable and effective method for the quantitative determination of HbA1c.
References
[1] HbA1c: A Review of Analytical and Clinical Aspects. Ann Lab Med 2013; 33:393-400.
[2] Consensus statement on the worldwide standardization of the hemoglobin A1C measurement: the American Diabetes Association, European Association for the Study of Diabetes, International Federation of Clinical Chemistry and Laboratory Medicine, and the International Diabetes Federation. Diabetes Care. 2007 Sep;30(9):2399-400. doi: 10.2337/dc07-9925.
[3] 2019: CLIA proposed changes to PT acceptable limits
[4] CAP (College of American Pathologists) acceptable limit for grading of the target value in 2020