The burden of postoperative pulmonary complications (PPCs)
Every year, more than 230 million major surgical procedures requiring general or regional anaesthesia are undertaken worldwide[1].
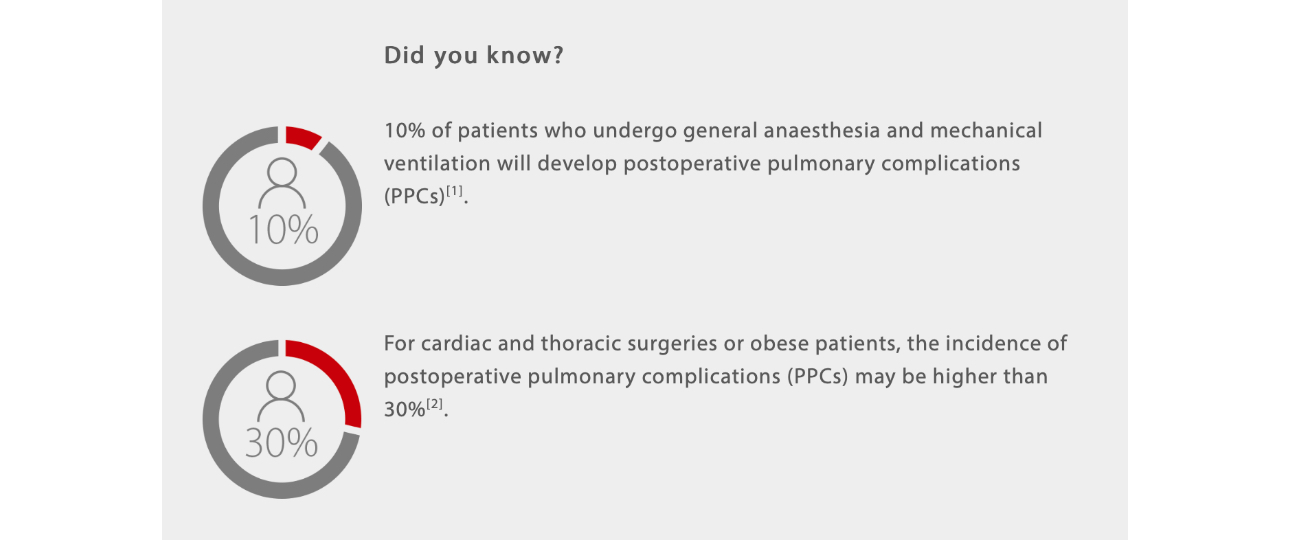
During the intraoperative stage, while initiating anaesthesia, the patient's respiratory system will significantly change. Initially, patients will lose consciousness and the central respiratory drive will be depressed. Then, the use of neuromuscular blocking drugs will paralyze the respiratory muscles, which can lead to a significant decrease of Functional Residual Capacity (FRC) of 15-20%[3]. Decrease of FRC frequently causes atelectasis and is one of the most common respiratory changes contributing to PPCs during general anaesthesia.
Atelectasis may develop in nearly 90% of patients under general anaesthesia and can persist throughout the postoperative period and even up to several days after surgery[4].
To prevent atelectasis, at times a higher tidal volume is used to recruit collapsed alveoli, however, this may lead to overdistension or even volutrauma[5,6]. Furthermore, injury of shearing forces from the repetitive opening and collapse of the alveoli, called "atelectrauma", can induce or worsen existing lung injuries.
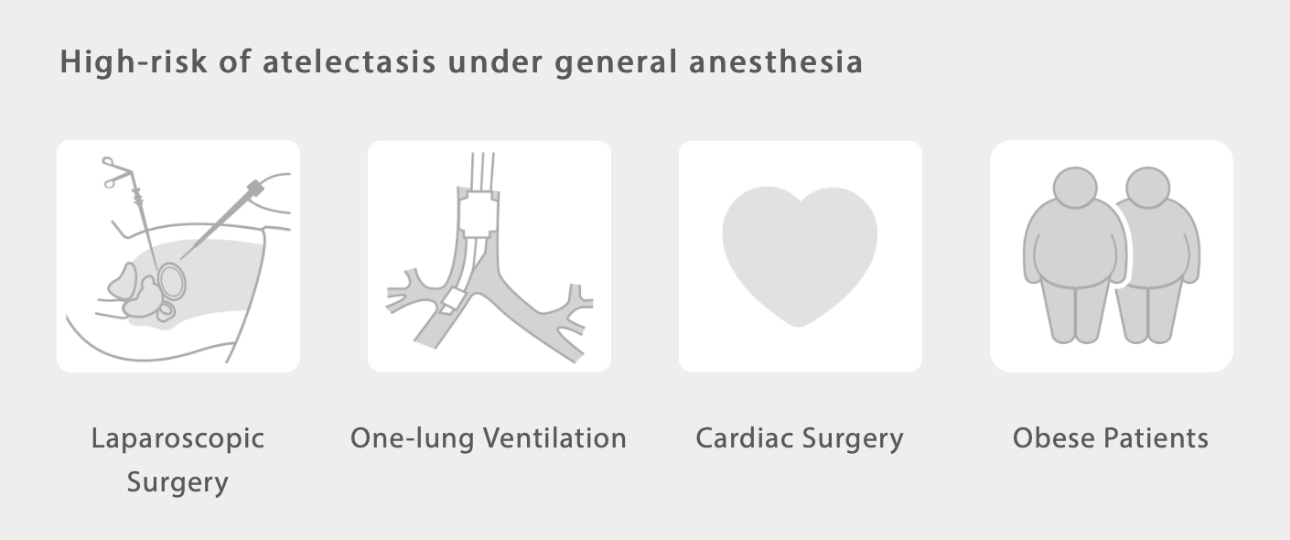
Lung protective ventilation is widely recognized as an effective strategy to keep the alveoli open while avoiding barotrauma.
Commonly used strategies include low-tidal volume, optimal PEEP titration, and lung recruitment maneuvers[7,8].
Protective Ventilation Strategies applied to anaesthesia system
Lower tidal volume
The IMPROVED clinical trial suggested that protective lung ventilation and non-protective ventilation have different tidal volume settings of 6-8ml/kg versus 10-12 ml/kg. In retrospect, the lung protective ventilation group has a lower risk of PPCs with lower levels of ventilation support required and shorter hospital stays after surgery[9].
However, in a recent randomized clinical trial among adults undergoing major surgery, low tidal volume strategy did not significantly reduce PPCs within the first 7 days after surgery if the PEEP setting is fixed to 5 cmH2O, suggesting clinical outcomes may not improve with only low tidal volume strategy[10].
Optimal PEEP for individualized patient care
A study on patients with a high risk of atelectasis, such as those who have undergone laparoscopic abdominal surgery, shows that individualized PEEP setting could reduce postoperative atelectasis while improving intraoperative oxygenation with minimum side effects[11,12]. The same result can be seen in obese patients, who require higher PEEP to keep the lungs open due to higher abdominal pressure[13].
Therefore, optimal PEEP titration during anaesthesia is recommended to improve lung function during and after surgery. The optimal PEEP is defined as the PEEP setting that leads to the lowest intrapulmonary shunt without compromising cardiac output[14]. But how is optimal PEEP titration for individual patients done?
There are several PEEP titration methods commonly used by clinicians, including: pulmonary compliance directed methods, best oxygenation, Vds/Vt guided technique based on imaging, and transpulmonary pressure directed procedures[11,12,15].
Recruitment maneuvers
Recruitment maneuver is a strategy aimed at re-expanding collapsed alveoli and augmenting the exchange surface at the alveoli-capillary membrane, to avoid intra-and postoperative lung complications[16].
So how is lung recruitment commonly done in the operating room? A wide variety of recruitment maneuvers have been reported and used in practice, including sustained inflation, stepwise increase of tidal volume ventilation, incremental PEEP procedure, etc. The best recruitment maneuver technique may vary according to the specific patients and circumstances[7,17].
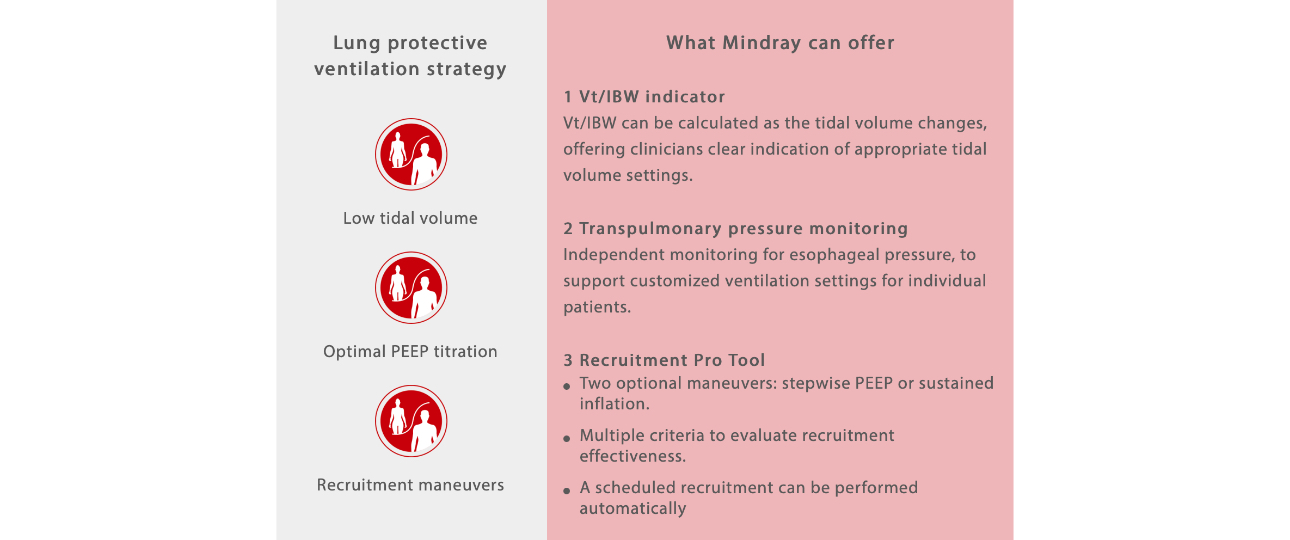
Striving for best practices towards patient safety in anaesthesia settings, Mindray incorporates various protective ventilation tools in the A8/A9 anaesthesia systems, which contributes to safer patient management by reducing the risk of PPCs and improving patient outcomes.
References:
[1] Thomas G Weiser, Scott E Regenbogen, Katherine D Thompson, Alex B Haynes, Stuart R Lipsitz, William R Berry, Atul A Gawande. An estimation of the global volume of surgery: a modelling strategy based on available data. Lancet 2008; 372: 139–44.
[2] Valentín Mazo , Sergi Sabaté, Jaume Canet, Lluís Gallart, Marcelo Gama de Abreu, Javier Belda, Olivier Langeron, Andreas Hoeft, Paolo Pelosi. Prospective external validation of a predictive score for postoperative pulmonary complications. Anesthesiology. 2014 Aug;121 (2):219-31.
[3] Lumb AB, Anaesthesia. In: AB. Lumb Nunn’s Applied Respiratory Physiology, 8th Edn. London: Elsevier, 2016; 291–318.
[4] H Lundquist , G Hedenstierna, A Strandberg, L Tokics, B Brismar. CT-assessment of dependent lung densities in man during general anaesthesia. Acta Radiol 1995; 36: 626–32.
[5] Dreyfuss D,et al. D Dreyfuss , P Soler, G Basset, G Saumon. High inflation pressure pulmonary edema. Respective effects of high airway pressure, high tidal volume, and positive end-expiratory pressure. Am Rev Respir Dis,1988,137:1159-1164.
[6] Davide Chiumello , Eleonora Carlesso, Paolo Cadringher, Pietro Caironi, Franco Valenza, Federico Polli, Federica Tallarini, Paola Cozzi, Massimo Cressoni, Angelo Colombo, John J Marini, Luciano Gattinoni.Lung stress and strain during mechanical ventilation for acute respiratory distress syndrome. Am J Respir Crit Care Med, 2008, 178:346–355.
[7] Andreas Güldner, Thomas Kiss, Ary Serpa Neto, et al., Intraoperative Protective Mechanical Ventilation for Prevention of Postoperative Pulmonary Complications, Anesthesiology 2015; 123:692-713.
[8] Emmanuel Marret, Raphael Cinotti, Laurence Berard, et al., Protective ventilation during anaesthesia reduces major postoperative complications after lung cancer surgery, Eur J Anaesthesiol 2018; 35:1–9.
[9] Emmanuel Futier, Jean-Michel Constantin, Catherine Paugam-Burtz, et al., A Trial of Intraoperative Low-Tidal-Volume Ventilation in Abdominal Surgery, N Engl J Med 2013;369:428-37.
[10] Dharshi Karalapillai, Laurence Weinberg, Philip Peyton et al., Effect of Intraoperative Low Tidal Volume vs Conventional Tidal Volume on Postoperative Pulmonary Complications in Patients Undergoing Major Surgery: A Randomized Clinical Trial, JAMA,. 2020 Sep 1;324 (9):848-858.
[11] Zoltán Ruszkai, Erika Kiss, Ildikó László, et al., Effects of intraoperative positive end-expiratory pressure optimization on respiratory mechanics and the inflammatory response: a randomized controlled trial, Journal of Clinical Monitoring and Computing 2020.
[12] Sérgio M. Pereira, Mauro R. Tucci, Caio C. A. Morais, et al., Individual Positive End-expiratory Pressure Settings Optimize Intraoperative Mechanical Ventilation and Reduce Postoperative Atelectasis, Anesthesiology 2018; 129:1070-81.
[13] Francis X. Whalen, Ognjen Gajic, Geoffrey B. Thompson, et al., The Effects of the Alveolar Recruitment Maneuver and Positive End-Expiratory Pressure on Arterial Oxygenation During Laparoscopic Bariatric Surgery, Anesth Analg 2006;102:298 –305.
[14] M.J. Civetta, T.A. Barnes, L.O. Smith“Optimal PEEP” and intermittent mandatory ventilation in the treatment of acute respiratory failure. Respiratory Care 1975; 20: 551–7.
[15] C. Nestler, P. Simon, D. Petroff, et al., Individualized positive end-expiratory pressure in obese patients during general anaesthesia: a randomized controlled clinical trial using electrical impedance tomography, Br J Anaesth. 2017 Dec 1;119(6):1194-1205.
[16] L. Magnusson, D. R. Spahn, New concepts of atelectasis during general anaesthesia, British Journal of Anaesthesia 2003, 91 (1): 61-72.
[17] Paolo Pelosi, Marcelo Gama de Abreu and Patricia RM Rocco, New and conventional strategies for lung recruitment in acute respiratory distress syndrome, Critical Care 2010, 14:210.