Mindray has reinforced its capacity for independent development and production of IVD raw materials with the acquisition of HyTest, a leading global provider of antibodies and antigens situated in Finland. The strategic acquisition has significantly bolstered Mindray's capability to provide cutting-edge immunoassay solutions in various domains, with a particular focus on cardiac disease management.
Strengthened Scientific Research Capabilities in Cardiac Biomarkers
With nearly 30 years of experience, HyTest specializes in developing and supplying world-class antibodies and antigens, particularly in the field of cardiac biomarkers for myocardial infarction and heart failure. Since its foundation in 1994, HyTest has been at the forefront of cardiac biomarker research. In the same year, HyTest introduced its first-generation cardiac troponin I monoclonal antibody, gaining recognition for its exceptional performance. [1-2]
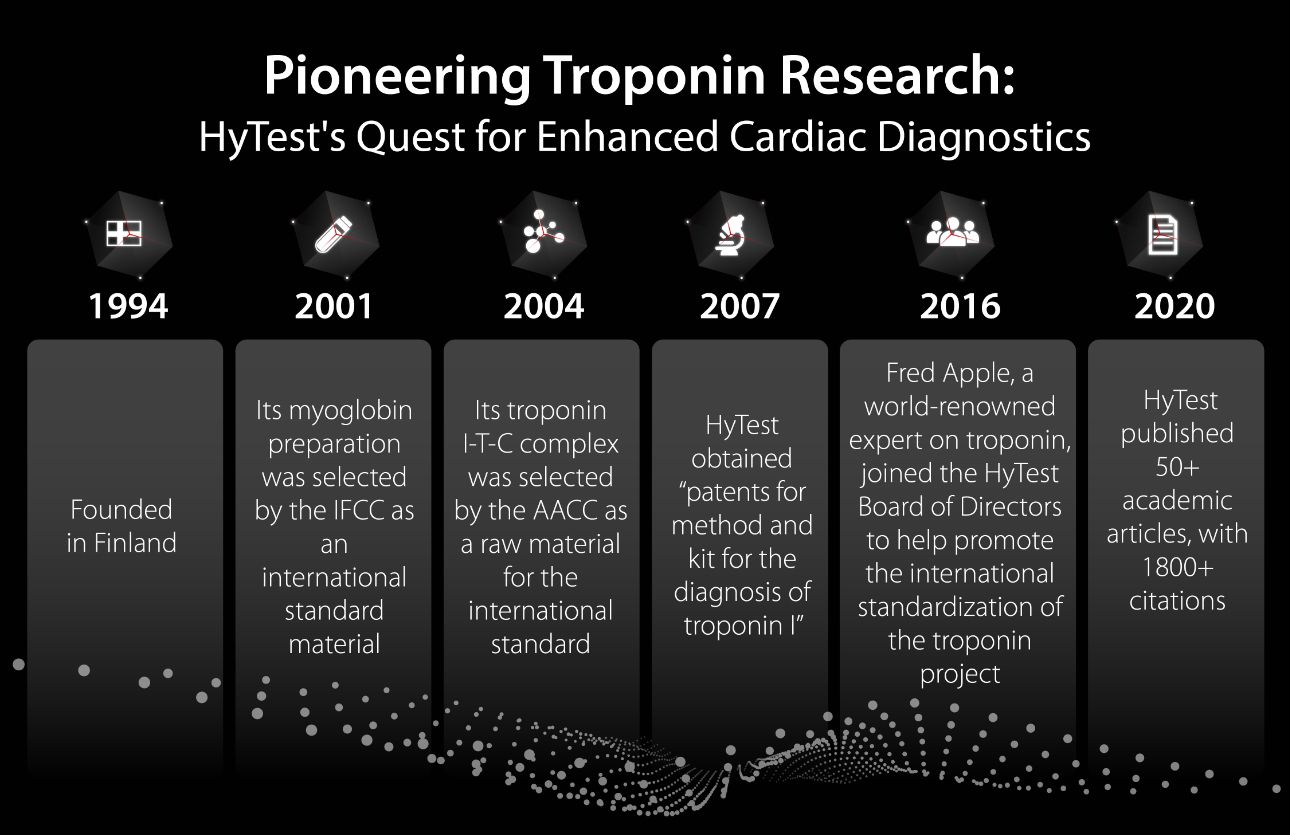
By harnessing the scientific expertise of HyTest alongside Mindray's innovative prowess, we have successfully developed the state-of-the-art cardiac assay, hs-cTnI (high-sensitivity troponin I). This achievement allows us to provide a wide range of cardiac tests in the immunoassay field, catering to the diverse needs of clinical settings.
hs-cTnI: Empowering Heart Risk Assessment
hs-cTnI, an exceptional assay, earns the "high-sensitivity" label for its ability to detect troponin I in over 50% of seemingly healthy individuals while maintaining a coefficient of variation below 10% at the 99th percentile upper-reference limit (URL). This remarkable sensitivity makes hs-cTnI an essential tool for diagnosing, stratifying risk, and managing acute coronary syndrome (ACS), a subset of cardiovascular diseases (CVDs). CVDs are a leading cause of death worldwide and impose a significant burden on many countries. As per WHO data, [3] CVDs accounted for 32% of global fatalities in 2019.
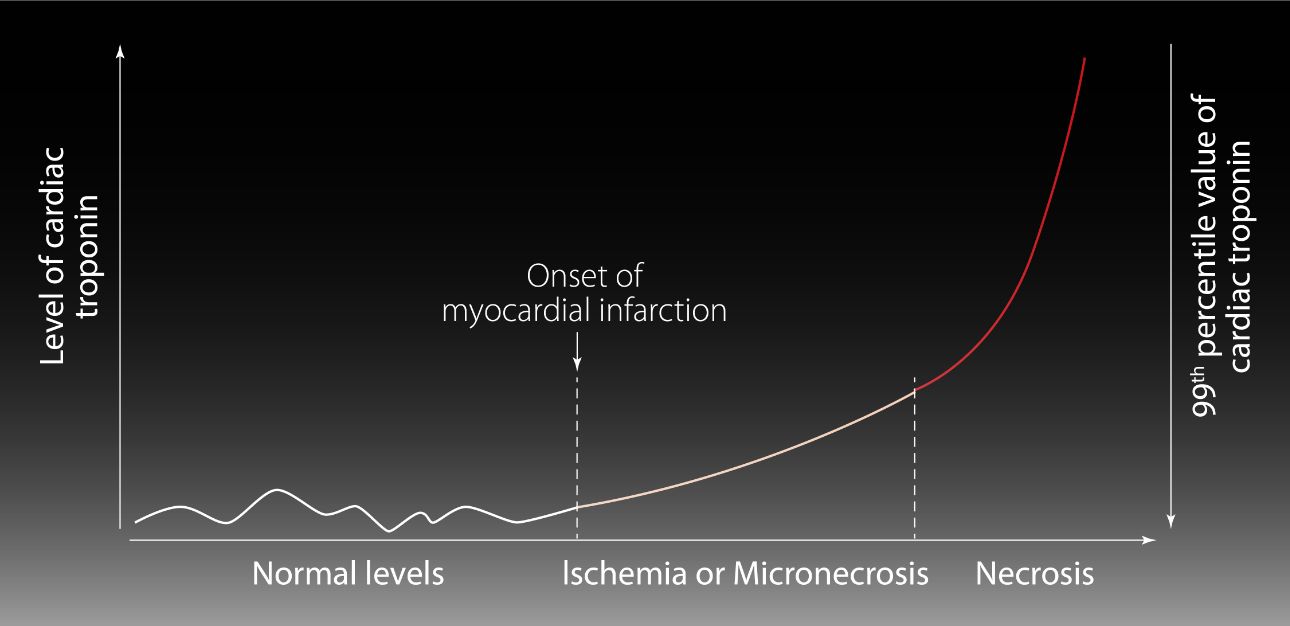
Identification of patients with AMI
Acute Myocardial Infarction (AMI) is a significant clinical manifestation of ACS. Per AMI guidelines, [4] the identification of individuals with AMI involves continuous monitoring of hs-cTnI values. This includes observing fluctuations in the values, with at least one measurement surpassing the 99th percentile URL. Moreover, clinical evidence indicating myocardial ischemia is essential for accurate identification.
Early diagnosis and rule-out/rule-in of NSTEMI
hs-cTnI testing not only aids in identifying individuals with a high likelihood of AMI but also facilitates the early diagnosis and rapid exclusion of AMI in a significant proportion of patients.
Since 2015, the European Society of Cardiology (ESC) has recommended employing the 0/1h algorithm (preferred) or the 0/2h algorithm (alternative) for swift rule-out and rule-in of Non-ST-segment-elevation myocardial infarction (NSTEMI) type of AMI. Numerous studies conducted in recent years have consistently demonstrated that hs-cTnI values falling below the level of detection yield a negative predictive value (NPV) for AMI exceeding 99%. [5-6]
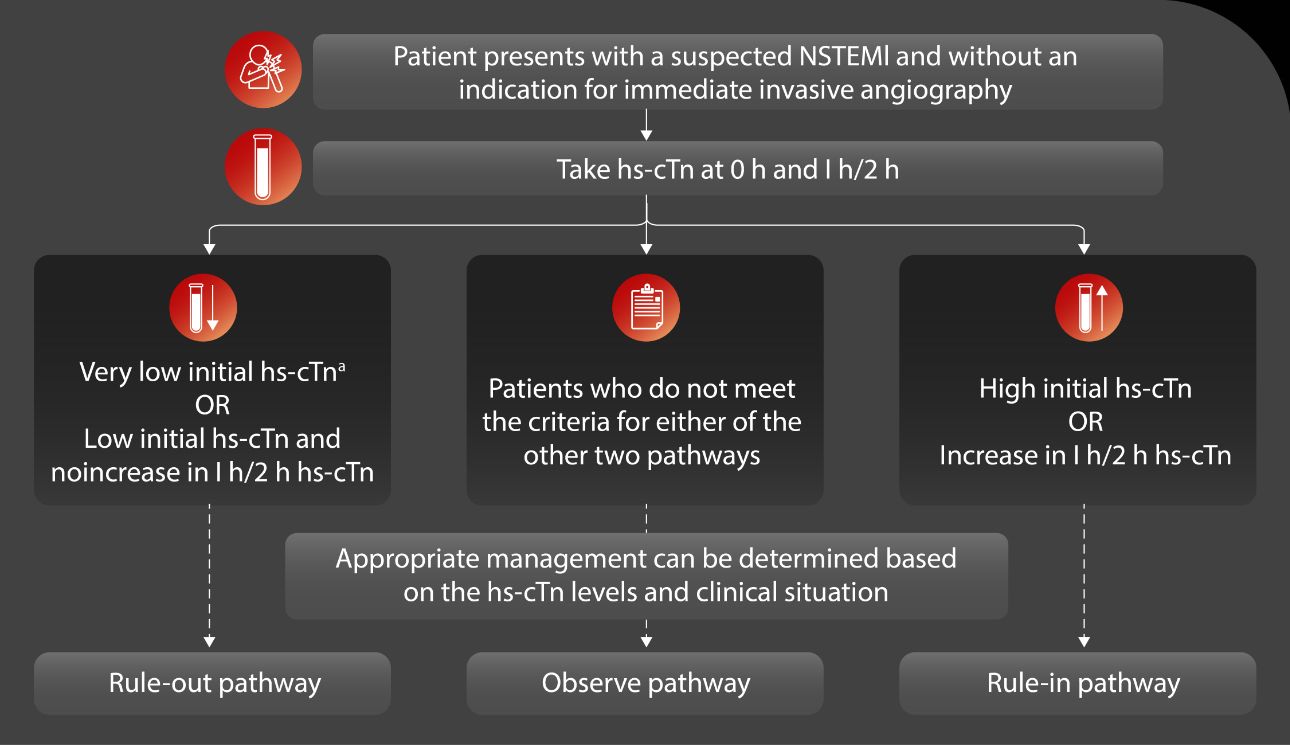
Differential diagnosis of myocardial injury
Elevated levels of hs-cTnI can also be detected in various other clinical conditions, including acute myocardial injury and chronic myocardial injury.
Similar to the diagnostic interpretation for AMI, acute myocardial injury may require at least one hs-cTnI concentration exceeding the 99th percentile URL, accompanied by a significant dynamic rise or fall pattern. [8] The critical clinical distinction between these two conditions is the absence of myocardial ischemia symptoms in acute myocardial injury.
Risk stratification and prognosis of patients with ACS
In addition to its diagnostic value in cases of AMI and myocardial injury, serial measurement of hs-cTnI levels, when combined with existing risk score systems, proves helpful in risk stratification for patients with suspected ACS who are receiving in-hospital care. It also provides valuable prognosis information in terms of short- and long-term mortality. An individual may be considered at intermediate risk based solely on a risk score even if hs-cTnI concentrations are below the 99th percentile. In contrast, the low-risk group can be characterized by hs-cTnI levels that are close to or below the limit of detection (LOD). [9]
Combining HyTest's advancements in cardiac biomarker research with Mindray's robust R&D capabilities in IVD, we are poised to introduce more cutting-edge products that provide reliable test results for clinical diagnosis. Mindray remains committed to aligning our research with clinical needs and translating scientific breakthroughs into practical applications in the management of cardiac and other diseases.

References
[1] Hytest cardiac markers brochure
[2] Hytest troponin booklet
[3] https://www.who.int/news-room/fact-sheets/detail/ cardiovascular-diseases-(cvds)
[4] Holzmann M J. Clinical implications of high-sensitivity cardiac troponins [J]. Journal of internal medicine, 2018, 284(1): 50–60.
[5] Thygesen K, Alpert J S, Jaffe A S, et al. Fourth Universal Definition of Myocardial Infarction (2018) [J]. Circulation, 2018, 138(20): e618-e651.
[6] McCord J, Hana A, Cook B, et al. The role of cardiac testing with the 0/1-hour high-sensitivity cardiac troponin algorithm evaluating for acute myocardial infarction [J]. American Heart Journal, 2021, 233: 68-77.
[7] Byrne R A, Rossello X, Coughlan J J, et al. 2023 ESC Guidelines for the management of acute coronary syndromes: Developed by the task force on the management of acute coronary syndromes of the European Society of Cardiology (ESC) [J], European Heart Journal, 2023, 00: 1–107.
[8] Sandoval Y, Apple F S, Mahler S A, et al. High-sensitivity cardiac troponin and the 2021 AHA/ACC/ASE/CHEST/SAEM/SCCT/SCMR guidelines for the evaluation and diagnosis of acute chest pain [J]. Circulation, 2022, 146(7): 569-581.
[9] Jia X M, Sun W S, Hoogeveen R C, et al. High-sensitivity troponin I and incident coronary events, stroke, heart failure hospitalization, and mortality in the ARIC study [J]. Circulation, 2019, 139(23): 2642-2653.