HPLC: The Gold Standard in HbA1c TestingWhy This Technology Powers Global Diabetes Management
2025-04-29

In diabetes care, glycated hemoglobin (HbA1c) is the cornerstone for evaluating long-term blood glucose control [1]. Among the various detection methods, High-Performance Liquid Chromatography (HPLC) stands out as the globally recognized "gold standard" [2]. This article explains how HPLC works, its advantages over other techniques, and why it remains indispensable in clinical practice.
How HPLC Works: Precision at the Molecular Level
HPLC detects HbA1c through a sophisticated separation process. When a blood sample is introduced into the system, red blood cells are lysed to release hemoglobin. The hemoglobin mixture then enters a column packed with specialized resin. Here, HbA1c, due to its unique charge and size resulting from glucose binding, is separated from non-glycated hemoglobin (e.g., HbA0). It's like different athletes on different tracks, ending up at the finish line at different times. As the separated components pass through a detector, their absorbance is measured, and software calculates the HbA1c percentage automatically. The entire process takes less than five minutes with minimal manual intervention, ensuring high throughput and reproducibility.
HPLC vs. Other HbA1c Detection Methods
While alternatives exist, HPLC’s superiority is evident. For instance, immunoassay, which relies on antibody-antigen binding, often suffers from cross-reactivity with hemoglobin variants like HbS or HbC, leading to inaccurate results. In contrast, HPLC’s physical separation method eliminates such interference, ensuring precise detection even in the presence of abnormal hemoglobin.
Another common method, enzymatic assay, uses enzyme-mediated reactions to measure HbA1c. However, this approach requires strict calibration and struggles with accuracy at low concentrations. HPLC, on the other hand, bypasses enzymatic variability entirely, relying instead on the inherent properties of hemoglobin molecules for separation.
Capillary electrophoresis, which separates hemoglobin components using an electric field, offers high resolution but is time-consuming and lacks the automation of HPLC. The latter’s fully automated workflow not only speeds up the process but also reduces the risk of human error, making it ideal for high-volume laboratories [3,4].
| Method | Principle | Limitations | HPLC's Edge |
|---|---|---|---|
| Immunoassay | Antibody-antigen binding | Cross-reactivity with Hb variants (e.g., HbS, HbC) | No antibody interference; detects variants simultaneously |
| Enzymatic Assay | Enzyme-mediated reaction | Requires strict calibration; poor low-concentration accuracy | Physical separation eliminates enzymatic variability |
| Capillary Electrophoresis | Electric field separation | Time-consuming; low automation | Fully automated; rapid results |
Global Impact of HPLC in Diabetes Care
HPLC’s precision and reliability have made it a cornerstone of diabetes management worldwide. Clinicians rely on HbA1c levels to guide treatment adjustments, as they reflect a patient’s average blood glucose over the past two to three months. HPLC’s ability to detect hemoglobinopathies - such as sickle cell disease - during routine testing further enhances its clinical utility, ensuring accurate results even in complex cases.
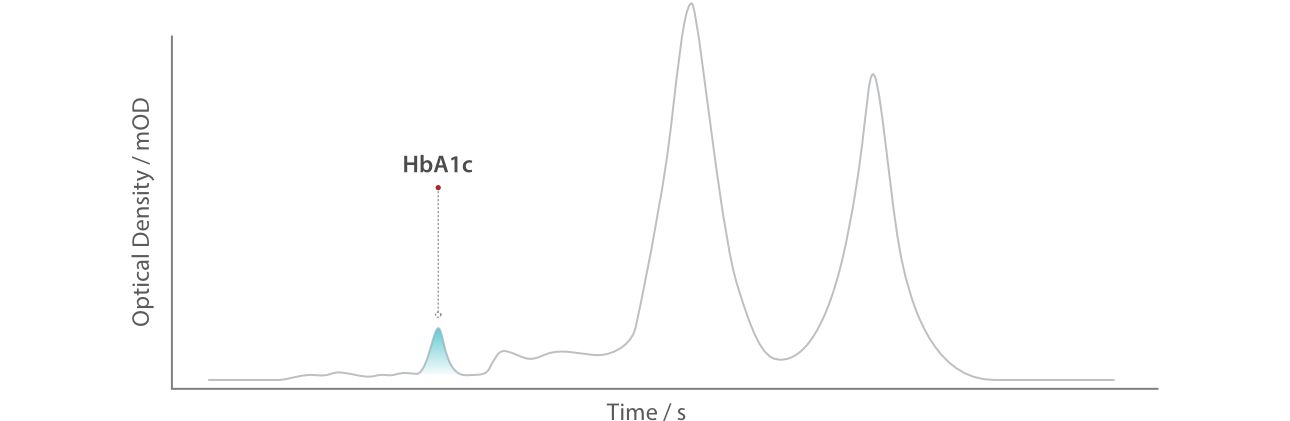
Beyond individual patient care, HPLC plays a critical role in public health. Its standardized methodology supports large-scale screening programs, enabling early detection and intervention in diverse populations. Moreover, HPLC aligns with guidelines from organizations like the WHO [5], NGSP [6] and IFCC [7] ensuring consistency across laboratories and facilitating global clinical trials.
Conclusion: The Unmatched Superiority of HPLC
HPLC’s blend of precision, speed, and adaptability makes it irreplaceable in diabetes care. By delivering standardized, Anti-interference results, it empowers clinicians to make informed decisions and supports global efforts to combat diabetes. As technology evolves, HPLC continues to set the benchmark - as the golden standard of HbA1c detection.
References
[1]. Sherwani, S. I., Khan, H. A., Ekhzaimy, A., Masood, A., & Sakharkar, M. K. (2016). Significance of HbA1c Test in Diagnosis and Prognosis of Diabetic Patients. Biomarker Insights, 11, 95-104. DOI: 10.4137/BMI.S38440
[2]. American Diabetes Association (ADA). (2023). Standards of Medical Care in Diabetes—2023. Diabetes Care, 46 (Supplement_1), S1-S291. DOI: 10.2337/dc23-Srev
[3]. Weykamp, C. (2013). HbA1c: A Review of Analytical and Clinical Aspects. Annals of Laboratory Medicine, 33(6), 393-400. DOI: 10.3343/alm.2013.33.6.393
[4]. Lenters-Westra, E., & Slingerland, R. J. (2010). Six of Eight Hemoglobin A1c Point-of-Care Instruments Do Not Meet the General Accepted Analytical Performance Criteria. Clinical Chemistry, 56(1), 44-52. DOI: 10.1373/clinchem.2009.130310
[5]. World Health Organization (WHO). (2011). Use of Glycated Haemoglobin (HbA1c) in the Diagnosis of Diabetes Mellitus.
[6]. Little, R. R., et al. (2001). The National Glycohemoglobin Standardization Program: A Five-Year Progress Report. Clinical Chemistry, 47(11), 1985-1992. DOI: 10.1093/clinchem/47.11.198
[7]. Jeppsson, J. O., et al. (2002). Approved IFCC Reference Method for the Measurement of HbA1c in Human Blood. Clinical Chemistry and Laboratory Medicine, 40(1), 78-89. DOI: 10.1515/CCLM.2002.016