Clinical Significance
Derived from megakaryocytes, platelets (PLT) are produced and matured in the bone marrow. Besides widely known thrombosis and wound repair, PLT also plays important roles in inflammation, immunity and cancer biology[1]. Normal reference intervals of PLT in peripheral blood varies in the range of 150-400×109/L. When the PLT value is lower than 100×109/L, a common clinical problem identified as thrombocytopenia (low PLT) may be the result[2].
There are several causes of thrombocytopenia including decreased PLT production, increased PLT destruction, increased splenic sequestration and dilution[3]. Currently, complete blood count (CBC) and blood smear reviews are essential diagnostic methods for the initial evaluation of thrombocytopenia samples[2]. Therefore, counting low PLT correctly by an auto hematology analyzer might be a prospective approach which will greatly reduce the blood smear rate, and save laborious time in the diagnostic lab, rapidly screening out thrombocytopenia samples.

However, counting low PLT correctly is not a simple process. How does Mindray’s high-end auto-hematology analyzer deal with low PLT samples?
Mindray Solution
Tech 1. Highly specific fluorescent staining
Fluorescent staining dye (FR dye) has been specially designed with the ability to identify target cells immediately. Next, the fluorescent molecule captures nucleic acid in PLT cells while avoiding interferences from small RBCs, RBC /WBC fragments and other small particles. PLT stained with specific fluorescent dye subsequently goes through the laser detector for optical measurement. The high specificity and affinity of fluorescent dye ensures that the binding inside PLT cells is stable enough for cells to flow through the laser. This ensures that even low amounts of PLT can be mapped and counted in the SF CUBE 3D scattergram accurately.
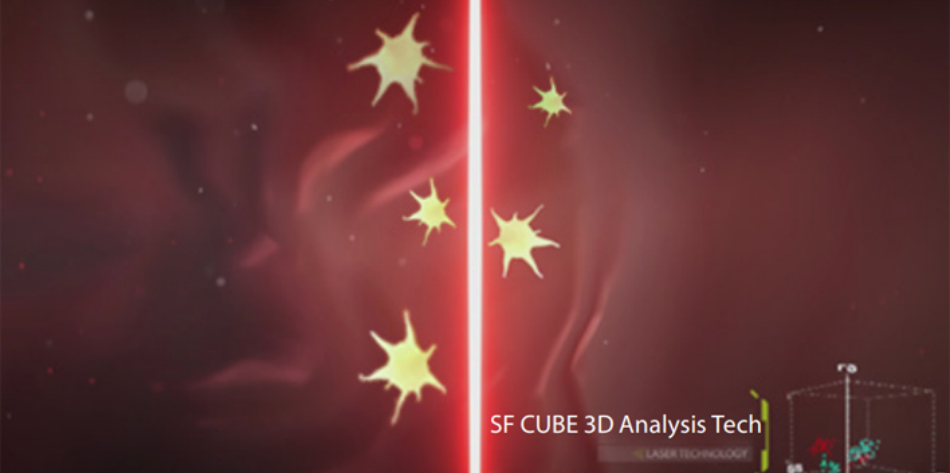
Tech 2. High-resolution optical detection
Also, Mindray’s high-end hematology analyzer combines reflective light suppression technology with SiPM (Silicon photomultiplier), which is highly sensitive to fluorescence signals while simultaneously minimizing the background noise during optical detection. This greatly improves the detection limit of particles, and the lower limit for small particles reaching up to 1um (a diameter of 2 um is defined as small PLT), ensuring that the sample results are not interfered by small PLT or particles.
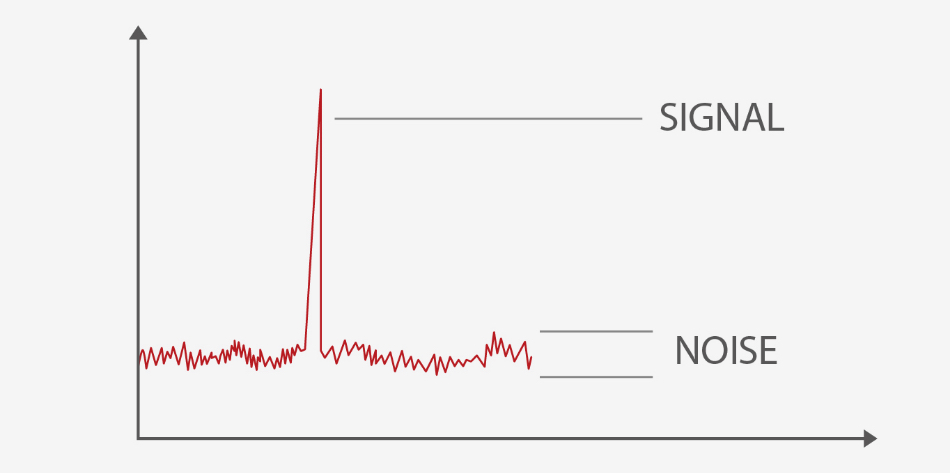
Tech 3. PLT de-aggregation
Pseudothrombocytopenia is caused by in vitro platelet clumping in EDTA-anticoagulated blood tube, which may lead to a falsely low PLT count[4]. Mindray's R&D conducted preliminary studies focusing on PLT's activation mechanisms, which is mainly regulated by calcium channel, tyrosine kinase pathway and GPIIb/IIIa. And then antagonists are applied in DR dilluent to directly block the binding sites on the surface of PLT which greatly minimizes the formation of PLT clumps. More detailed PLT de-aggregation mechanism will be released in the next HemaBook Chapter.
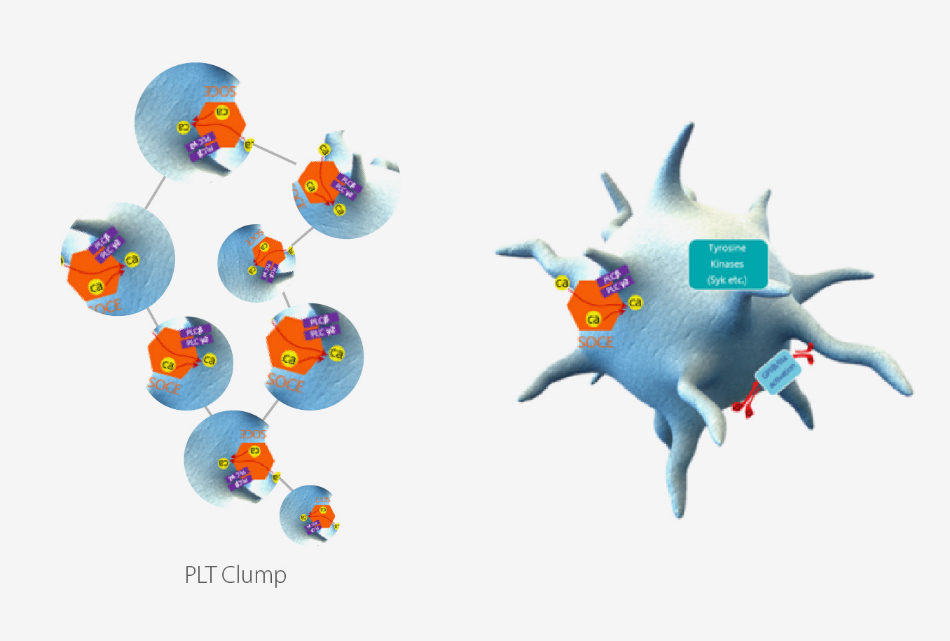
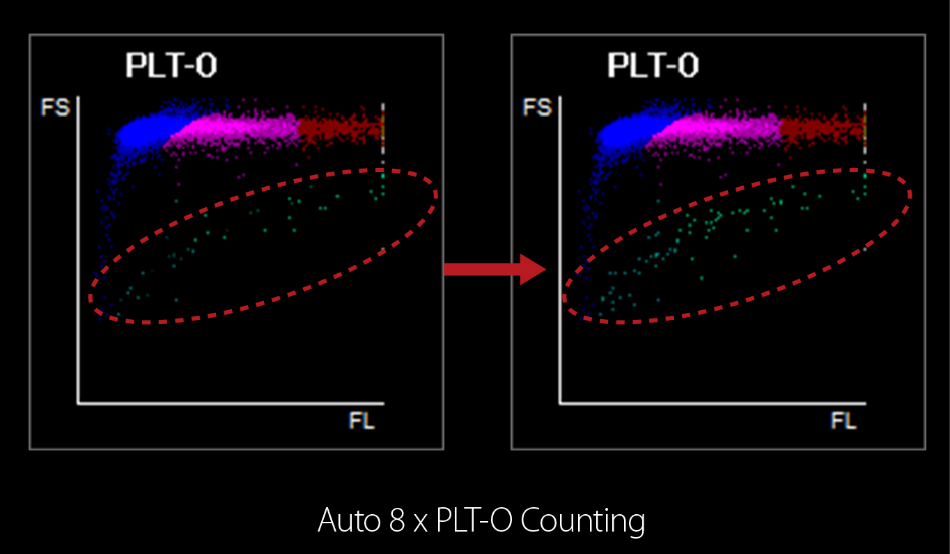
Figure 4. Auto 8×PLT-O counting
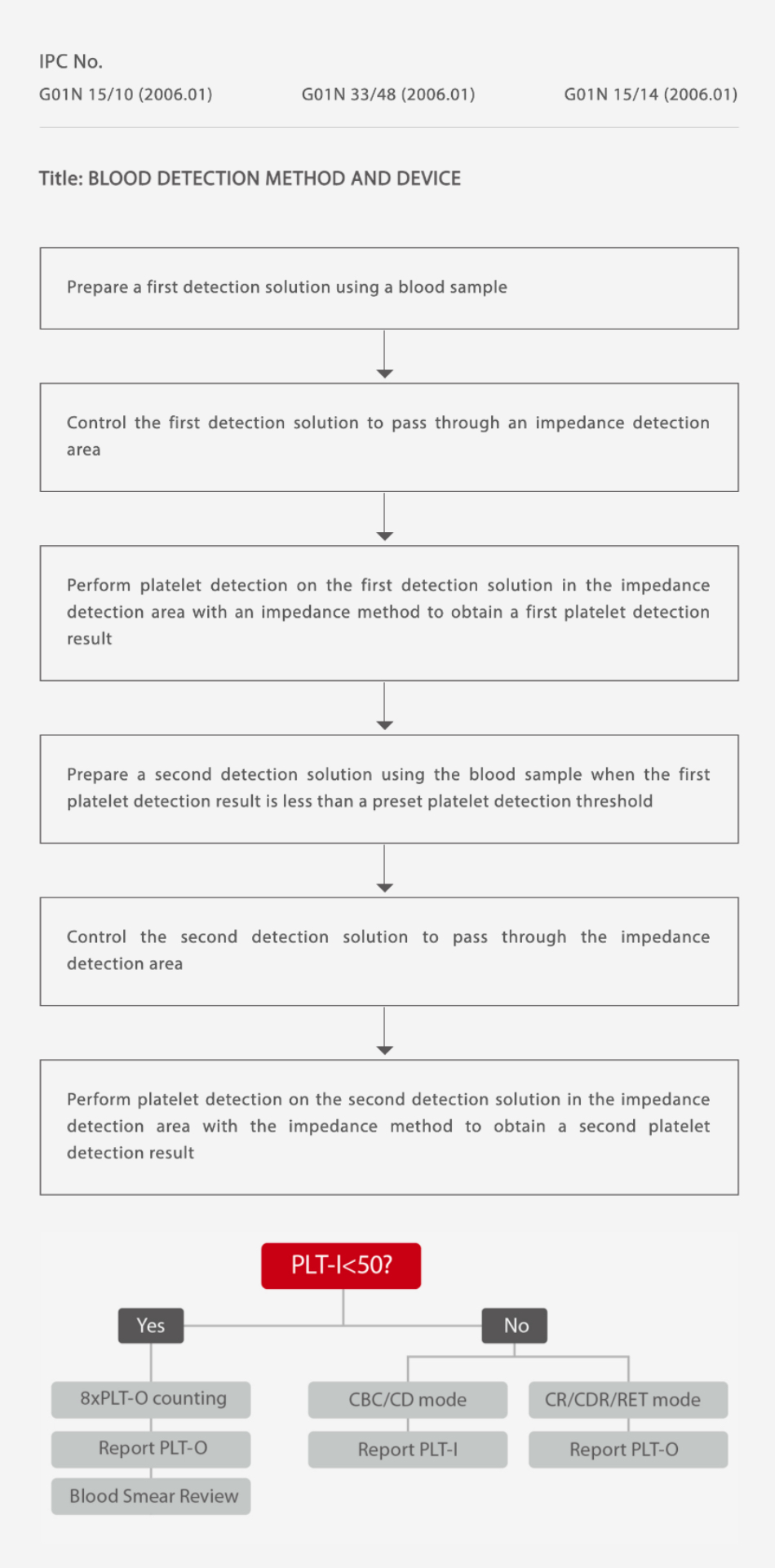
Mindray is in the process of a patent application (see below picture) for Auto 8×PLT-O Counting Technology. To begin with, PLT-I value obtained from impedance channel is firstly compared with a default value (50×109/L). If this is lower than the cutoff value, the analyzer can automatically prolong counting time up to 8-fold in order to collect more PLT particles for further analysis. In addition, Auto 8×PLT-O Counting Tech can eliminate other interference factors (e.g. fragmented RBC/WBC) which are often easily miscounted as PLT by the impedance channel. No additional sampling, no manual intervention, no additional channels and reagents required – 8×PLT-O counting is both efficient and effective in counting low PLT correctly.
References:
[1] Thrombocytopenia, Eun-Ju Lee, et al, Prim Care Clin Office Pract 43 (2016) 543–557.
[2] Platelet disorders: an overview, M. Krishnegowda, et al, Blood Coagulation and Fibrinolysis, 2015, Vol 26 No 5.
[3] Thrombocytopenia: an update, K. J. Smock, et al, Int. Jnl. Lab. Hem. 2014, 36, 269–278.
[4] Pseudothrombocytopenia, M Blonska, et al, Wiad Lek. 2001;54(5-6):333-6.