The quality of healthcare is a growing concern worldwide. In clinical laboratories, it is essential to define the quality required by an analytical testing process.
It is known that Sigma metrics are a useful tool for benchmarking the performance of methods.
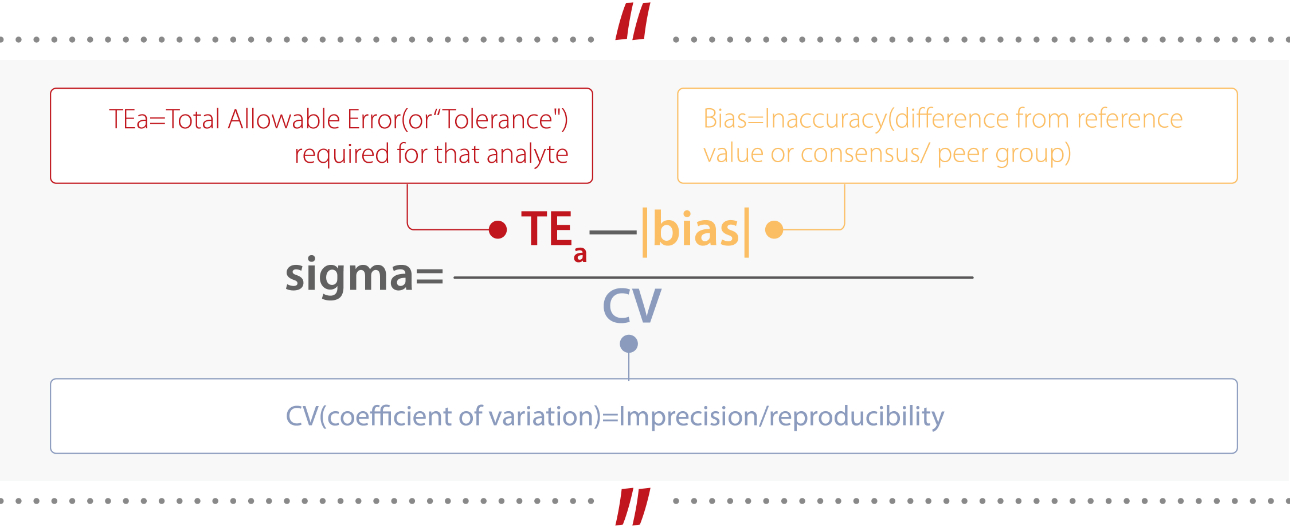
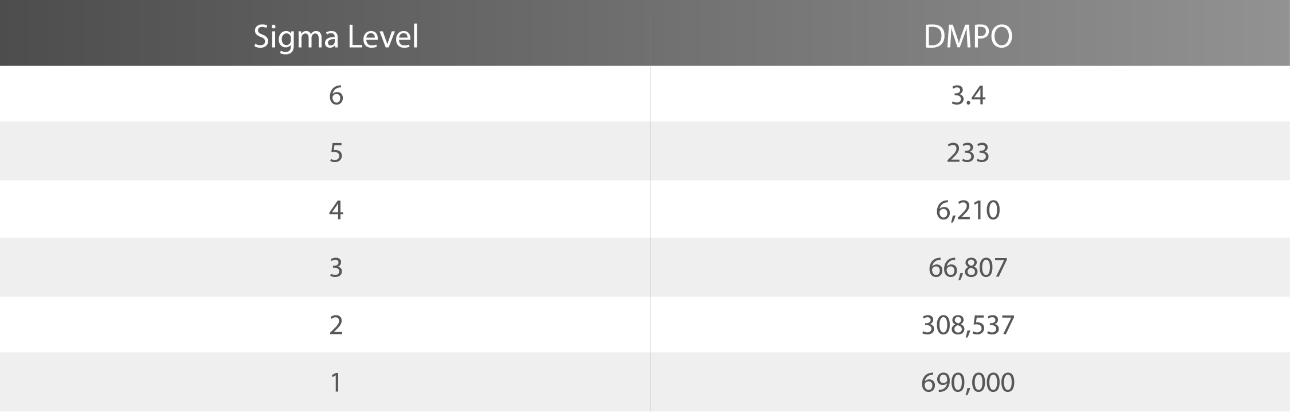
In the six Sigma methodology, “Defects per Million Opportunities (DPMO)” is used as the unit for calculation. A Sigma value indicates the frequency of defects occurring in a process.
Therefore, a higher Sigma value translates into a lower number of defects and a lower Sigma value means a higher number of defects. (Table 1)[1].
Below is a Sigma method decision chart, where the y-axis indicates the inaccuracy (bias, trueness) and the x-axis indicates the imprecision (CV).
Superimposed on this graph are the Sigma metrics zones:
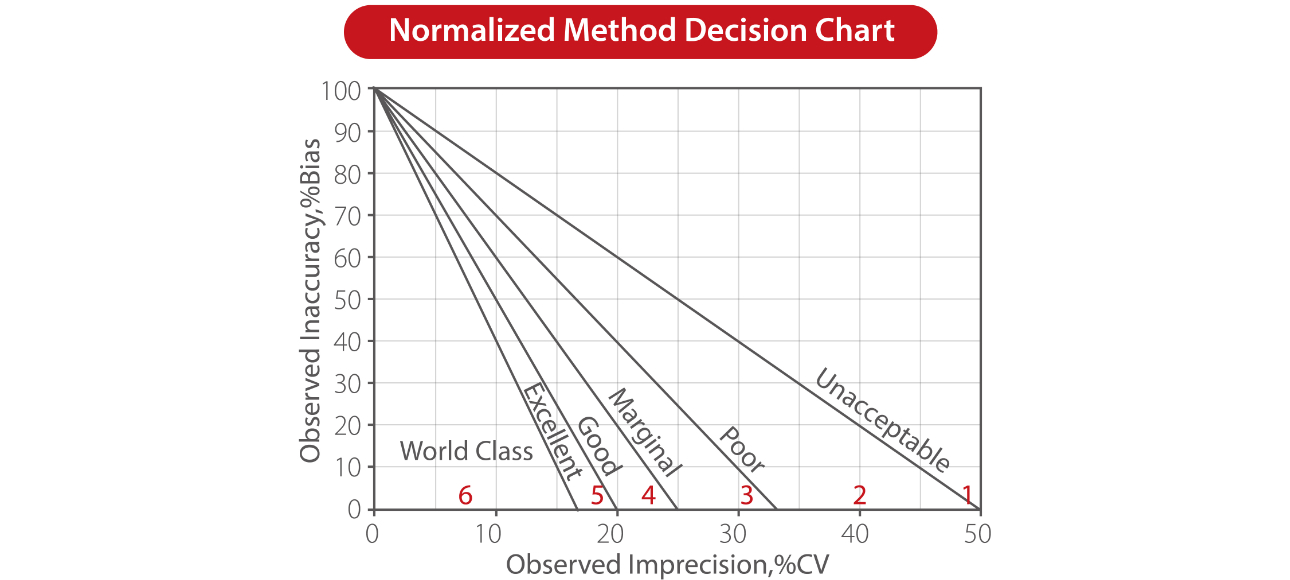
- Six Sigma zone (world class quality)
- Five Sigma zone (Excellent)
- Four Sigma zone (Good)
- Three Sigma zone (Marginal)
- Two Sigma zone (Poor)
- One Sigma zone (Unacceptable)
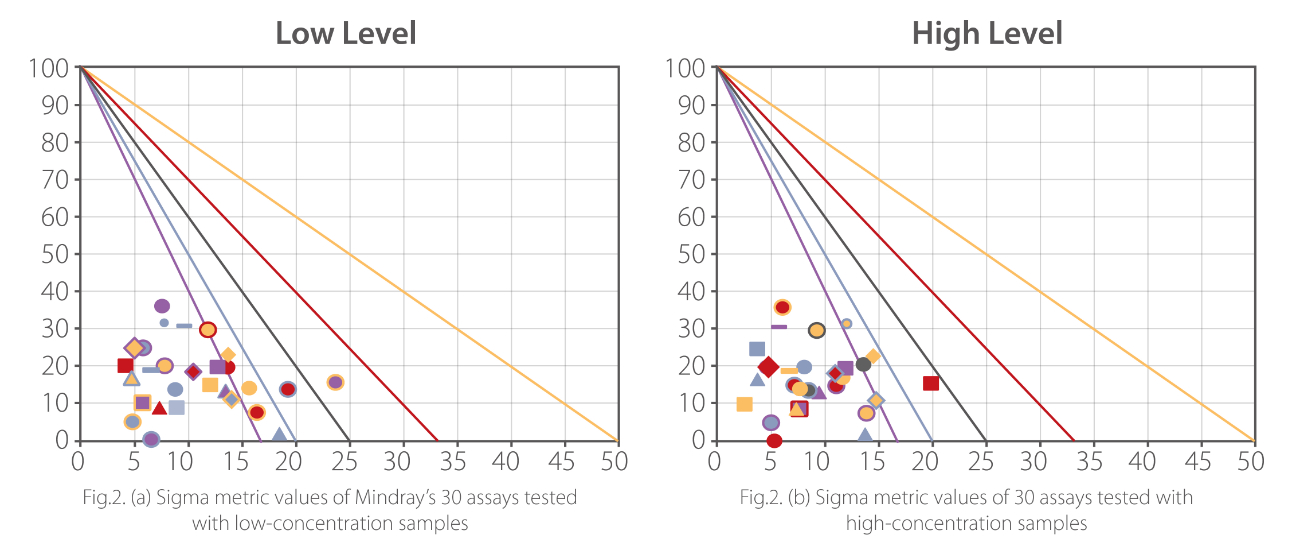
Mindray R&D used the Sigma method decision chart to plot the performance of 30 biochemistry parameters on BS-600M. The detailed performance of each parameter is shown in Fig.2 and Table 2.
The majority (around 75%) of the plotted performance falls in the World Class or Six Sigma zone, and around 95% tests have six Sigma and five Sigma performance. On this chart, we can see that no tests show a performance of 3, 2 or less than 2 Sigma.
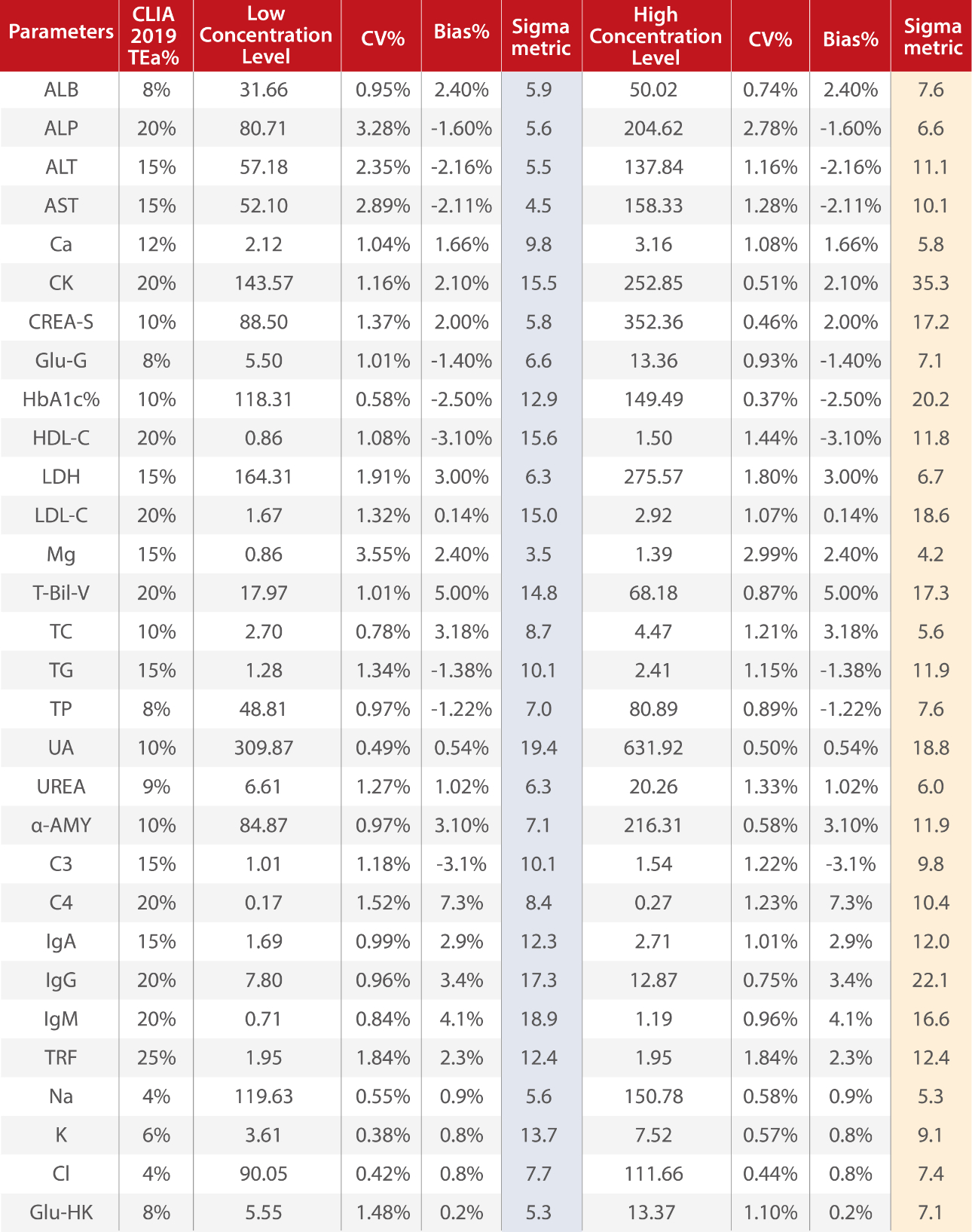
A high Sigma performance means high quality of measurement, which can improve clinicians’ confidence and satisfaction.
Besides performance benchmarking, Sigma metrics can also be used by laboratories to design quality control processes. Fig. 3 provides guidance on which control rules should be applied based on the Sigma quality determined in your laboratory[2].
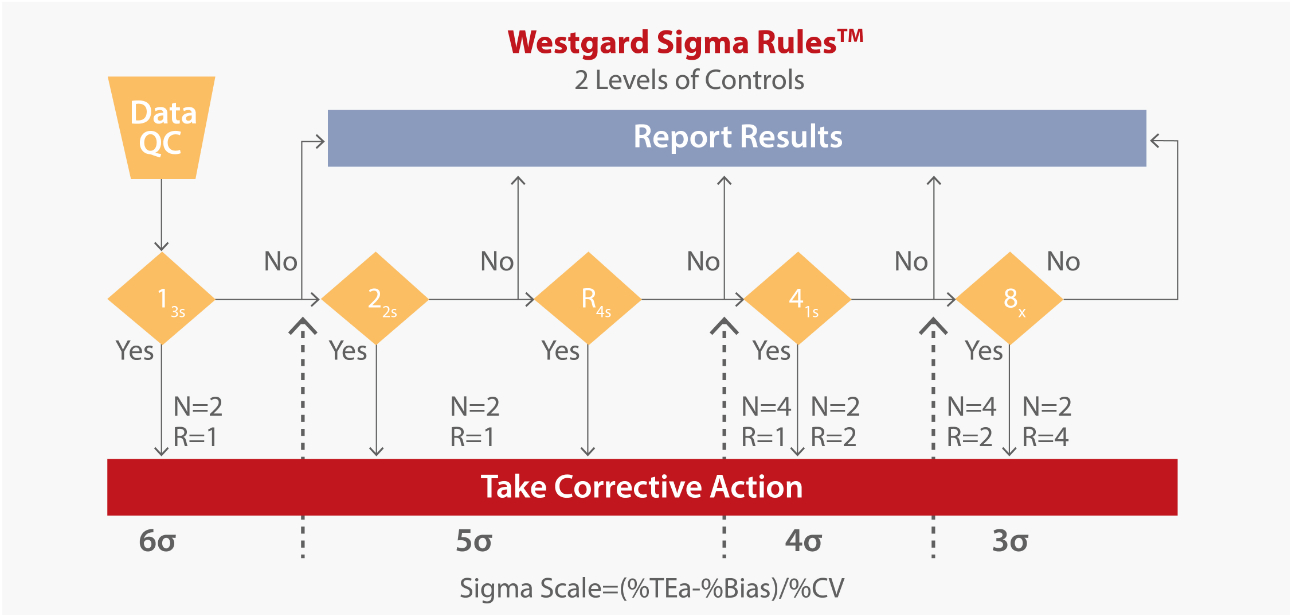
- 6-sigma quality requires only a single control rule, 13s, with 2 control measurements in each run. The notation N=2 R=1 indicates that 2 control measurements are needed in a single run.
- <4-sigma quality requires a multirule procedure that includes the 8x rule, which can be implemented with 4 control measurements in each of 2 runs (N=4, R=2) or alternatively with 2 control measurements in each of 4 runs (N=2, N=4). The first option suggests dividing a days’ work into 2 runs with 4 control measurements per run, whereas the second option suggests dividing a day’s work into 4 runs and monitoring each with 2 controls.
Sigma metrics can also be used as a guideline by laboratories for planning QC frequency accordingly[3].
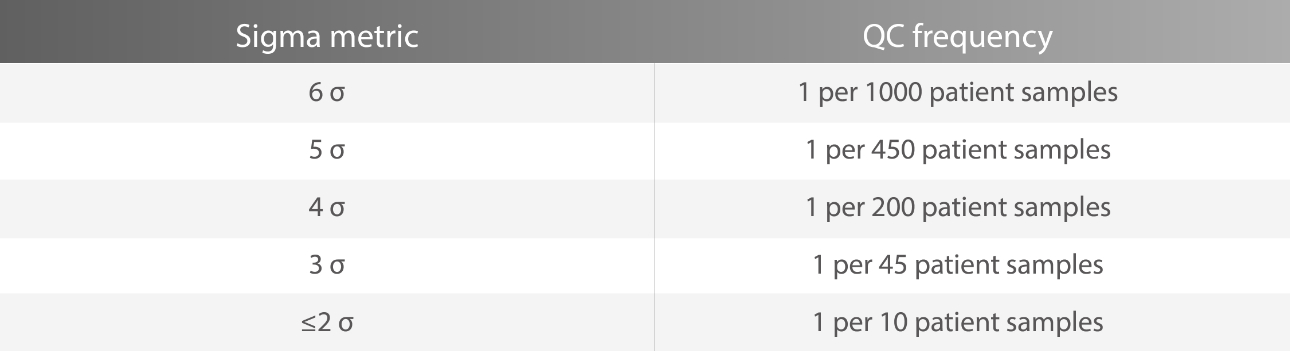
For assays with 6-sigma performance, the official recommendation is that laboratories can run QC every 1000 patient samples. For those with 3-sigma performance, it is recommended to run QC every 45 patient samples.
So, what does that mean? How does that impact the laboratory?
A high sigma performance level helps laboratories reduce QC measurements, simplify QC rules, and shorten the TAT. It also contributes to a reduced need for troubleshooting due to false QC violations and less QC and reagent consumption.
Therefore, with its high sigma level, Mindray’s BS-600M system can help laboratories offer high level of quality at reduced costs and boost clinicians’ confidence and laboratories’ reputation.
References
1. Kalra J, Kopargaonkar A. Quality improvement in clinical laboratories: A six sigma concept. Pathol Lab Med Open J. 2016; 1(1): 11-20.
2. Introducing Westgard Sigma Rules. https://www.westgard.com/westgard-sigma-rules.htm
3. Aggarwal K et al. Application of Six sigma metrics and Method decision charts in improvising clinical chemistry laboratory performance enhancement. Int J Adv Med. 2019 Oct;6(5):1524-1530

