Ultrasound Journal 14 - Basic and advanced ultrasonographic techniques for evaluation of prostatic artery embolization: One stop shopping
By Moschouris H, PhD,EBIR,FCIRSE and Stamatiou K, PhD 2023-06-12
Background
During the last decade, prostatic artery embolization (PAE) has emerged as a promising endovascular treatment for symptomatic benign prostatic hyperplasia (BPH), combining efficacy with an attractive safety profile and with the ability to be applied in poor surgical candidates.
Imaging studies (particularly ultrasound) are extensively used to provide valuable information before, during and after PAE [1,2]. Grey-scale US is a simple, flexible and widely available technique which is routinely utilized to measure prostatic volume (PV) before and after the procedure. Intravesical prostatic protrusion (IPP) and post-void residual volume (PVR) can also be easily evaluated with grey-scale US. Reduction of PV, IPP and PVR post PAE usually becomes evident one month or more post PAE, nevertheless changes of these parameters are useful measures of the effectiveness of PAE and correlate with clinical outcomes.
CEUS for Prostate Artery Embolization: Intraprocedural Monitoring and Efficacy Feedback
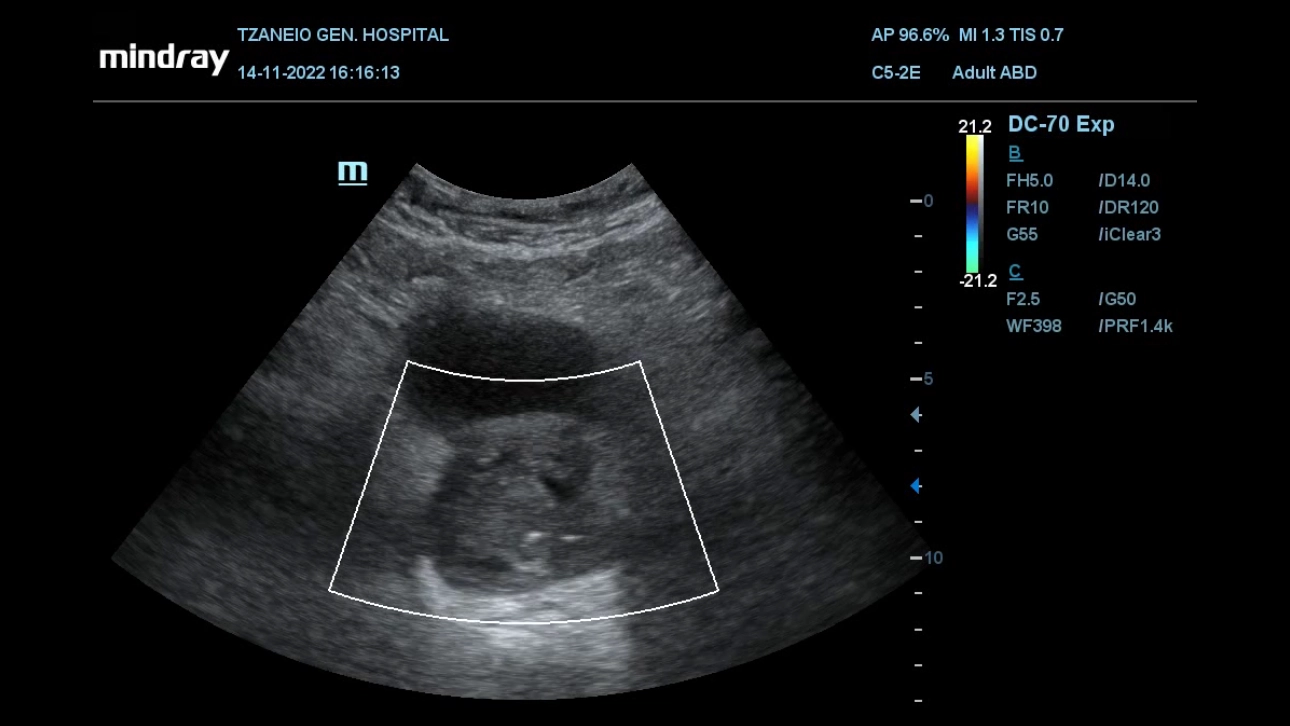
In addition to the aforementioned morphologic evaluation, contrast-enhanced US (CEUS) with intravenous administration of a second-generation echo-enhancer (SonoVue, Bracco, Milan, Italy) can be applied to study the hemodynamic and perfusional changes that occur in the prostate post PAE [3,4]. Prostatic infarcts can be easily detected as enhancement defects immediately post PAE (Fig 1); they are considered as a reliable sign of the local efficacy of PAE and they also correlate with clinical success.
Importantly, CEUS can be applied in the angio-suite, during the PAE procedure, preferably at the end of the injection of the embolic material (Fig 2) to provide immediate feedback on the efficacy of PAE [2]. If this on-site CEUS study shows limited or no prostatic infarction, the interventionalist may attempt to inject more embolic material in the selected artery or to investigate for additional arteries feeding the prostate. Another CEUS technique that can also be performed intraoperatively entails intraarterial administration of diluted echo enhancer through the microcatheter, in order to delineate the area perfused by the selected artery [2,5]. This appears to be an effective preventive measure of non-target embolization. For intraprocedural monitoring of PAE (and of other endovascular interventions) the authors prefer a portable unit (M8,Mindray), which is a standard part of the equipment of the angio-suite and which, despite its compact size, rapidly provides high quality gray scale, color doppler as well as CEUS imaging during PAE.
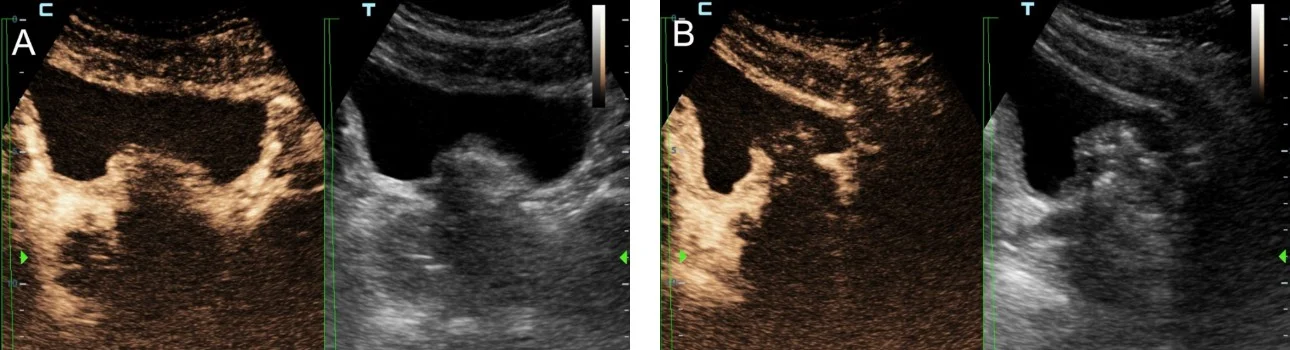
Shear-Wave Elastography for Quantifying Therapeutic Changes in Prostate Elasticity Post-PAE
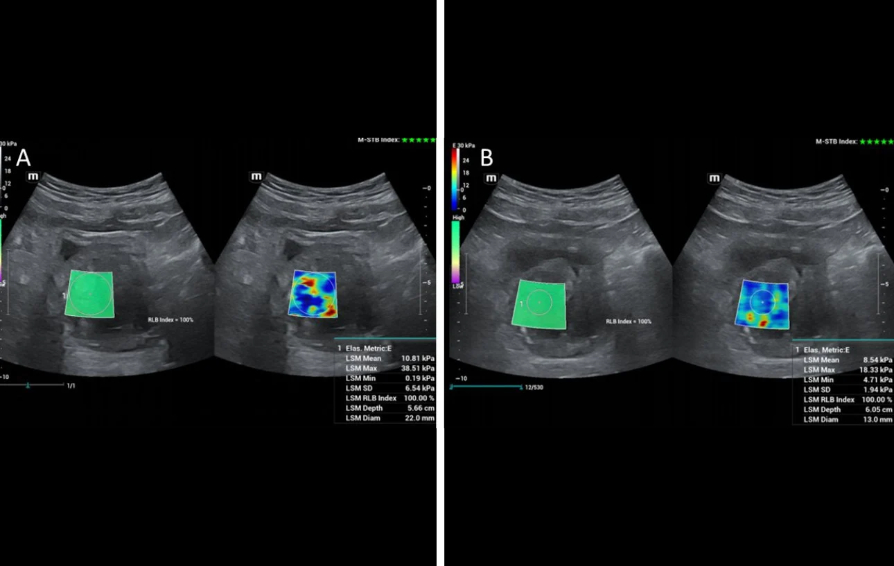
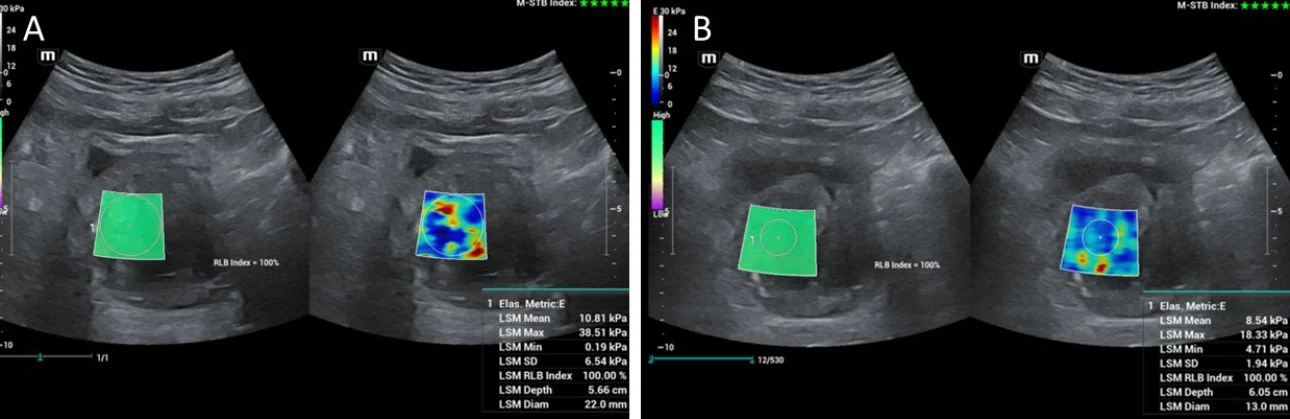
Shear-wave elastography has also been applied for the study of the prostate before and after PAE and remarkable changes in shear-wave velocity and in the elastic modulus have been recorded; US elastography can therefore detect and quantify therapeutic changes in elasticity of prostate tissue post PAE [6,7]. The respective studies have been performed with transrectal ultrasound [6,7, 8]; however initial experience at the institution of the authors indicates that, with appropriate equipment, transabdominal shear wave elastography of the prostate could also detect changes in prostate elasticity post PAE (Fig 3). This and other research purposes are served by a high-end machine (Resona I9, Mindray) with a wide variety of probes.
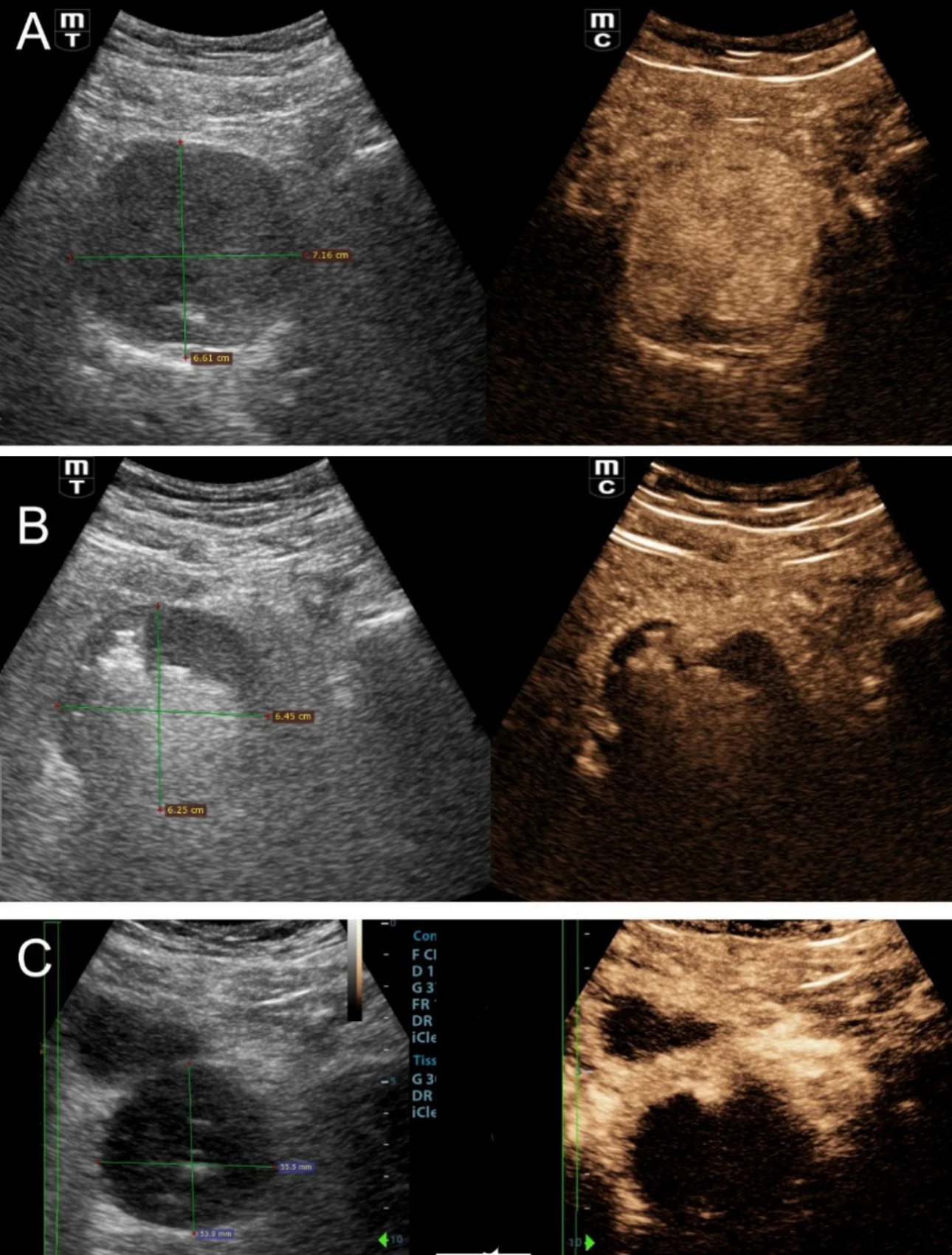
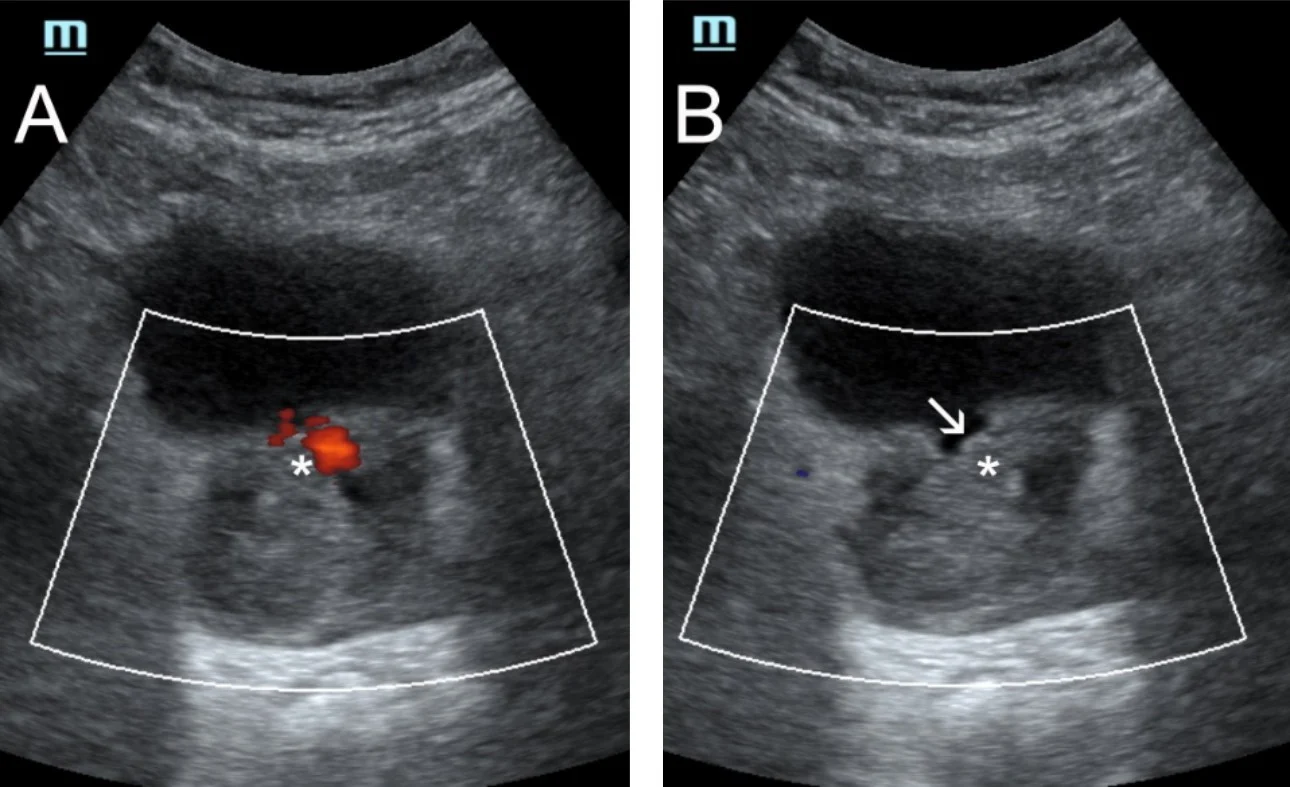
Finally, the authors utilize transabdominal US for non-invasive, dynamic study of urination in selected patients who experience symptomatic recurrence post PAE [9]. Interestingly, in a few of these patients, necrosis and degeneration of prostatic tissue post PAE, results in freely mobile prostatic remnants that intermittently protrude in the bladder outlet and/or in the urethra and block urine flow (Fig 4).
Conclusion
In conclusion, basic and advanced US techniques, thanks to their non-invasiveness, flexibility and high diagnostic yield, enable radiologists and urologists involved in PAE to study several aspects of this procedure and at multiple time points. The respective imaging findings can be used to increase safety and efficacy of PAE, for follow-up and for research purposes.
References
[1] Fernandes L, Rio Tinto H, Pereira J et al.Prostatic arterial embolization: post-procedural follow-up. Tech Vasc Interv Radiol. 2012;15:294-299
[2] Moschouris H, Dimakis A, Anagnostopoulou A et al. Sonographic evaluation of prostatic artery embolization: Far beyond size measurements. World J Radiol. 2020;12:172-183
[3] Moschouris H, Stamatiou K, Kalokairinou Motogna M et al.Early post-interventional sonographic evaluation of prostatic artery embolization. A promising role for contrast-enhanced ultrasonography (CEUS). Med Ultrason. 2018; 20:134-140
[4] Moschouris H, Stamatiou K, Malagari K et al. The value of contrast-enhanced ultrasonography in detection of prostatic infarction after prostatic artery embolization for the treatment of symptomatic benign prostatic hyperplasia. Diagn Interv Radiol. 2019;25:134-143
[5] Nzekwu E, Mirakhur A, Lee A et al. Intra-Arterial and Intravenous Contrast-Enhanced Ultrasonography in Prostate Artery Embolization: A Case Series. J Vasc Interv Radiol. 2018;29:1399-1402
[6] de Assis AM, Moreira AM, Carnevale FC et al. Effects of Prostatic Artery Embolization on the Dynamic Component of Benign Prostate Hyperplasia as Assessed by Ultrasound Elastography: A Pilot Series. Cardiovasc Intervent Radiol. 2019;42:1001-1007
[7] de Assis AM, Moreira AM, Carnevale FC et al.Role of Ultrasound Elastography in Patient Selection for Prostatic Artery Embolization.J Vasc Interv Radiol. 2021;32:1410-1416
[8] Moschouris H, Stamatiou K, Dimakis A et al. Re: de Assis et al. "Effects of Prostatic Artery Embolization on the Dynamic Component of Benign Prostate Hyperplasia as Assessed by Ultrasound Elastography: A Pilot Series". Cardiovasc Intervent Radiol. 2019;42:1366-1368
[9] Moschouris H, Stamatiou K, Spanomanolis N, et al. Degenerated, Pouch-Shaped, Intermittently Protruding Median Lobe Causing Early Symptomatic Recurrence After Prostatic Artery Embolization. Cardiovasc Intervent Radiol. 2022;45:1411-1414