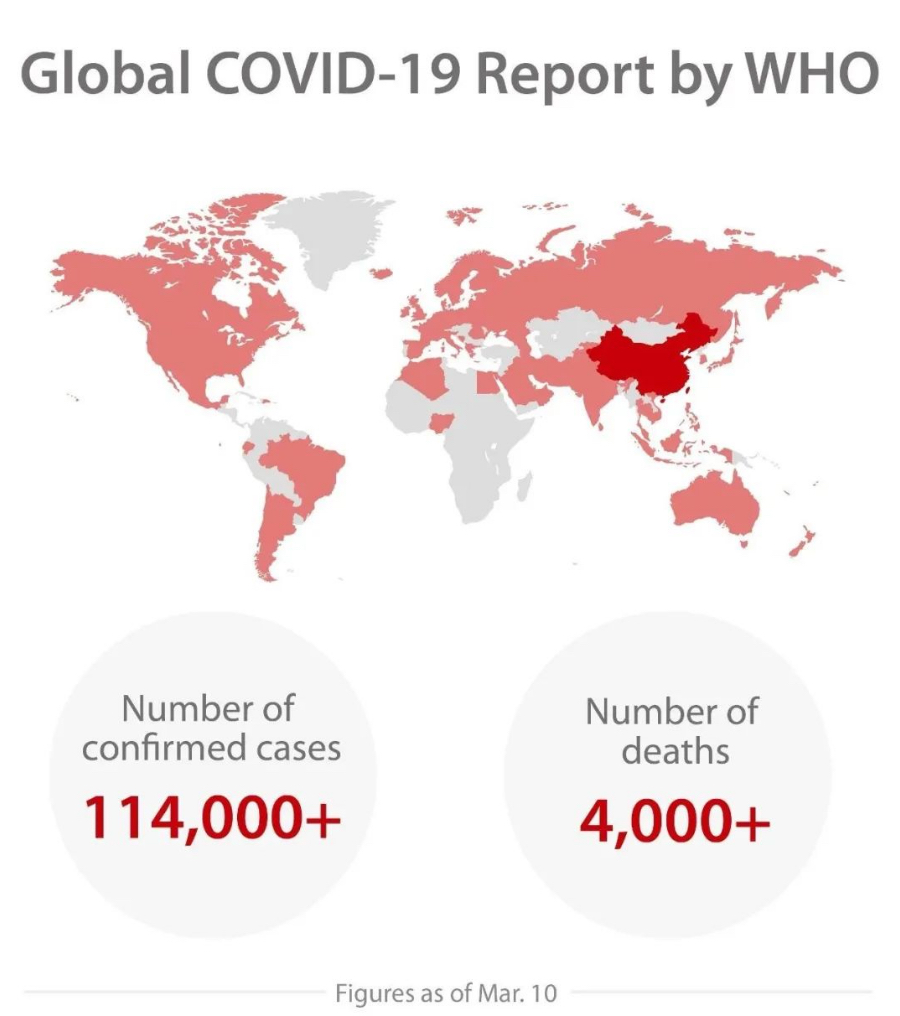
The current outbreak of COVID-19, a dangerous new coronavirus disease caused by the SARS-CoV-2 virus, has spread throughout China and 114 other countries and regions as of March 10. As the epidemic is evolving fast, healthcare workers around the world are racing against time to respond to the outbreak.
According to the the 6th Edition of the Provisional Criteria for Diagnosis and Treatment of COVID-19, laboratory examination on COVID-19 patients shows that in the early stage of infection, the total number of white blood cells (WBC) in the patient’s peripheral blood turns to be normal or below average, with a lower lymphocyte count. Most patients would show elevated C-reactive protein (CRP) with normal procalcitonin (PCT) values. Patients who are critically ill would show progressive decrease in peripheral blood lymphocytes.
Our laboratory team at the Jingzhou Central Hospital conducted a case study on a severe COVID-19 patient. We monitored all the parameters mentioned above throughout the whole process of the patient’s hospitalization (both diagnosis and treatment included). Laboratory testing results show that CBC and CRP values have changed exactly in line with the law of disease development. We share this case study in the hope that it will provide helpful reference for front-line doctors in evaluating and treating COVID-19 patients.
Basic Information and Test Results
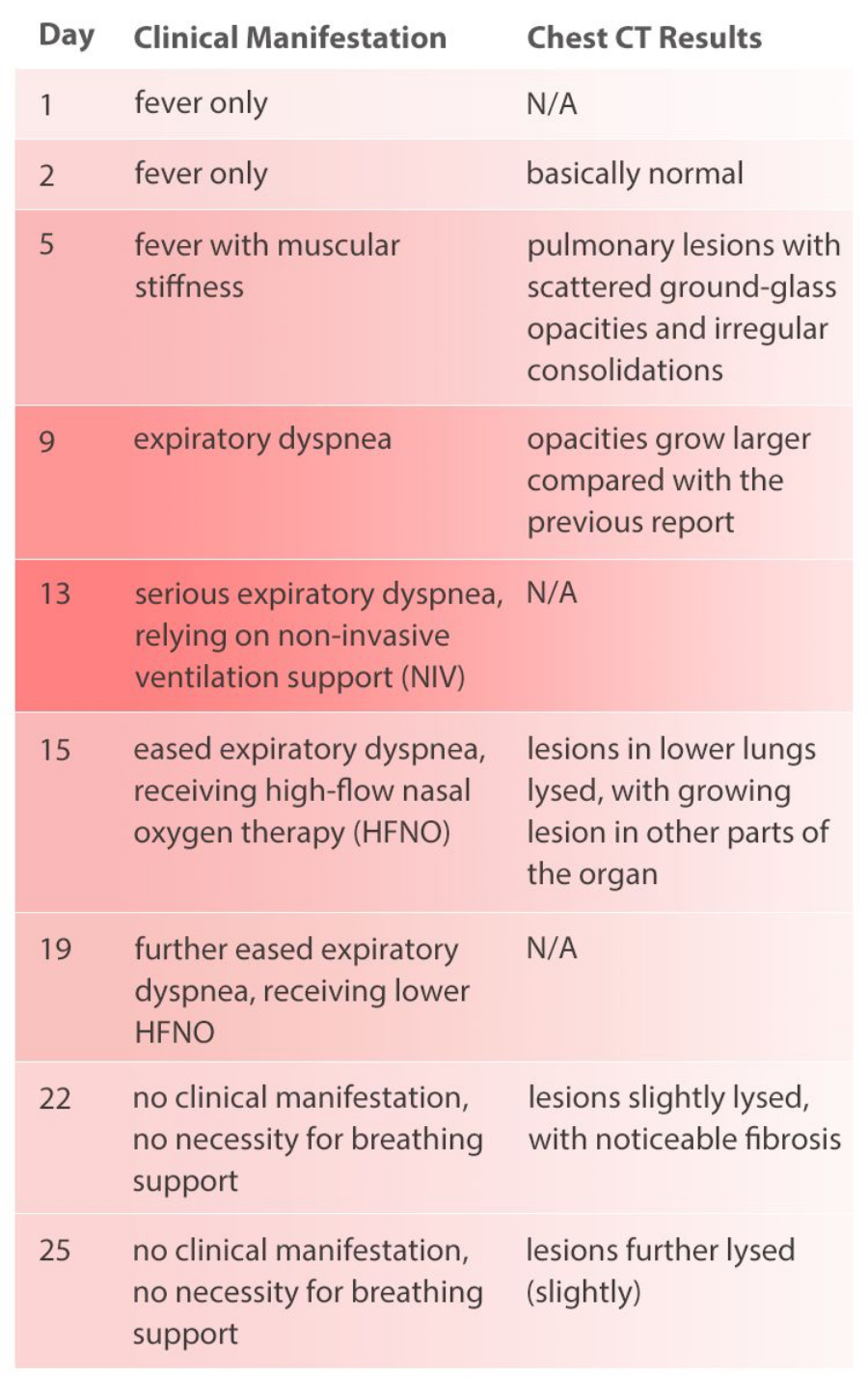
The patient being monitored in the study had a history of close contact with individuals from Wuhan. The patient registered low fever 5 days before admission with no other symptoms, and the CBC and CRP results were normal. Re-examination on the day after showed a slight decrease of WBC and a slight increase of CRP value. Levofloxacin tablets were taken orally following doctor's instruction for anti-infective treatment. On the day of admission, the patient had a high fever (maximum 39℃). Chest CT scan showed pulmonary lesions with scattered ground-glass opacities and irregular consolidations. The patient was given a presumptive diagnosis as a suspected case of COVID-19.
During the whole process of diagnosis and treatment, the patient’s clinical manifestations and chest CT scan results have been recorded (see Table 1), and so are CBC and CRP results (see Figure 1).
Discussion
Since the outbreak in December 2019, epidemiological investigations have found that transmission takes place mainly via respiratory droplets and close contact. Although it is not clear whether aerosol and digestive tract are also channels for transmission, people are generally all susceptible to the COVID-19. [1]
Most patients have moderate symptoms. However, with development of the disease, patients may exhibit fever, coughing, shortness of breath and dyspnea. Some patients may rapidly develop into acute respiratory distress (ARDS) or even die from multiple organ failure. [2]
The patient in this case got fever as the first symptom, with a clear epidemiological history (close contact with another person returning from Wuhan). Chest CT scan before admission suggested that this was probably a viral pneumonia patient who was later confirmed as a COVID-19 patient after nucleic acid test. After admission, the patient's breathing gradually worsened to respiratory failure. After mechanical ventilation and other treatments related, the clinical symptoms basically disappeared with improved imaging results. The patient was discharged after two negative results of nucleic acid test.
The patient had a low fever at the time of the initial examination, but the WBC count and lymphocyte count were normal, suggesting that the patient was in the early stage of COVID-19 at that time.
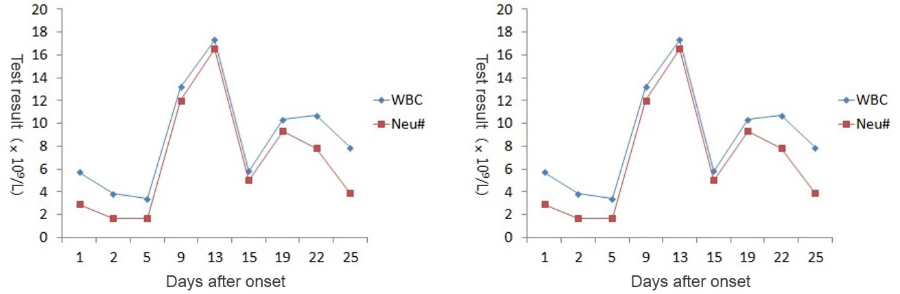
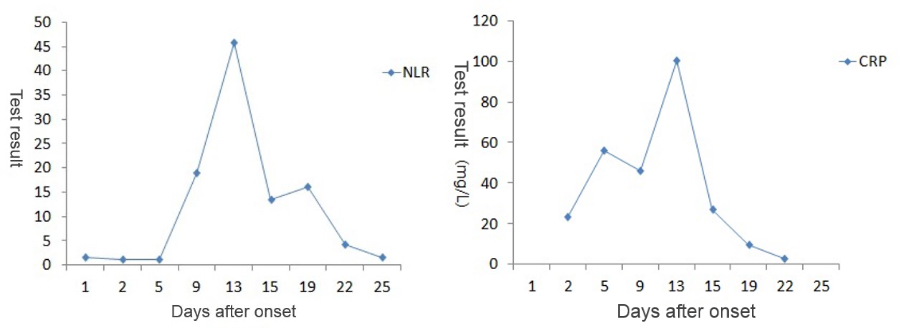
As the patient’s health deteriorated, the white blood cell count (WBC#) and neutrophil count (Neu#) increased sharply after a slight decline, while the lymphocyte count (Lym#) continued to decline. The Neutrophil-to-Lymphocyte Ratio (NLR) rose rapidly while the CRP value goes up. At the most critical moment (the 13th day after the onset of symptoms), WBC#, Neu#, NLR and CRP reached their peaks respectively, with Lym# at the lowest point.
After proper medical treatment and caring, the patient gradually recovered, with rapid decline of WBC#, Neu#, NLR and CRP. In contrast, Lym# gradually increased. On the 25th day, WBC#, Neu#, NLR, Lym# and CRP all returned to normal range. Although CT scan showed that the lesions in lungs hadn’t completely disappeared yet, the patient was already able to move without oxygen therapy. Most importantly, the nucleic acid test result turned negative.
A preprint of Neutrophil-to-Lymphocyte Ratio Predicts Severe Illness Patients with 2019 Novel Coronavirus in the Early Stage, a paper written by Beijing Ditan Hospital (affiliated to Beijing University of Medical Sciences) was published on the medRxiv platform on February 12. The study concludes that Neutrophil-to-Lymphocyte Ratio (NLR) can be used as the biomarker to predict the severity of COVID-19’s progression.a

An NLR greater than 3.13 can be used as an independent predictor for the exacerbation of patients with COVID-19. The finding of this case is consistent with that in the study published by Ditan Hospital. The patient’s NLR was higher than 3.13 from the 9th day to the 22nd day, and the NLR drops below 3.13 on the 25th day. In this case, PCT was monitored on Day 5, 13, and 22, all of which were lower than 0.5 ng/mL, indicating that no serious bacterial infection occurred.
Conclusion
The CBC and CRP change exactly in line with the law of disease development during the diagnosis and treatment of COVID-19 patients. Monitoring these routine laboratory test results, including WBC, Lym#, NLR and CRP, during the hospitalization period is helpful for managing patient’s medical treatment and prognosis.
About the author

References:
[1]. Chen N, Zhou M, Dong X, et al. Epidemiological and clinical characteristicsof 99 cases of novel coronavirus pneumonia in Wuhan, China: a descriptive study[J]. Lancet, 2020Jan30.pii:S0140-6736(20)30211-7. DOI:10.1016/S0140-6736(20)30211-7. [Epub ahead of print].
[2]. WANG M, CAO R, ZHANGL, et al. Remdesivir and chloroquineeffectively inhibit the recently emerged novel coronavirus (2019-nCoV) in vitro[J]. Cell Research, 2020.