
Value Chain Collaboration
Mindray always believe that the sustainability of an enterprise is inseparable from value chain collaborative management. We continue to maximize the value of end-to-end collaborative management via a management system covering R&D, procurement, manufacturing, sales and marketing, customer service and post-marketing supervision. Internally, the product quality and safety are guaranteed with total quality management system, which involves a variety of business functions. Externally, we, in partnership with external stakeholders along the value chain, reinforce suppliers and marketing management, jointly contributing to the sustainability of the enterprise.
Product quality and safety
We strictly abide by the applicable laws and regulations in the countries and regions where we operate, as we firmly believe that the product quality and safety are not only crucial to patient health, but also pivotal to achieving our mission and social responsibility. We have established a strict total quality management system and continuously refine it in line with the increasingly stringent regulatory requirements, under which we monitor product quality at every stage of our value chain, and ensure the product safety and stability with lean management.

As a healthcare technology company, the Group strictly complies with the regulations, standards, and requirements of the global sales regions, and establishes "Internally Developed Product Standard" for products in all products and manufacturing bases to ensure that the safety, performance and quality of each type of product meet the regulations, standards and customer needs.
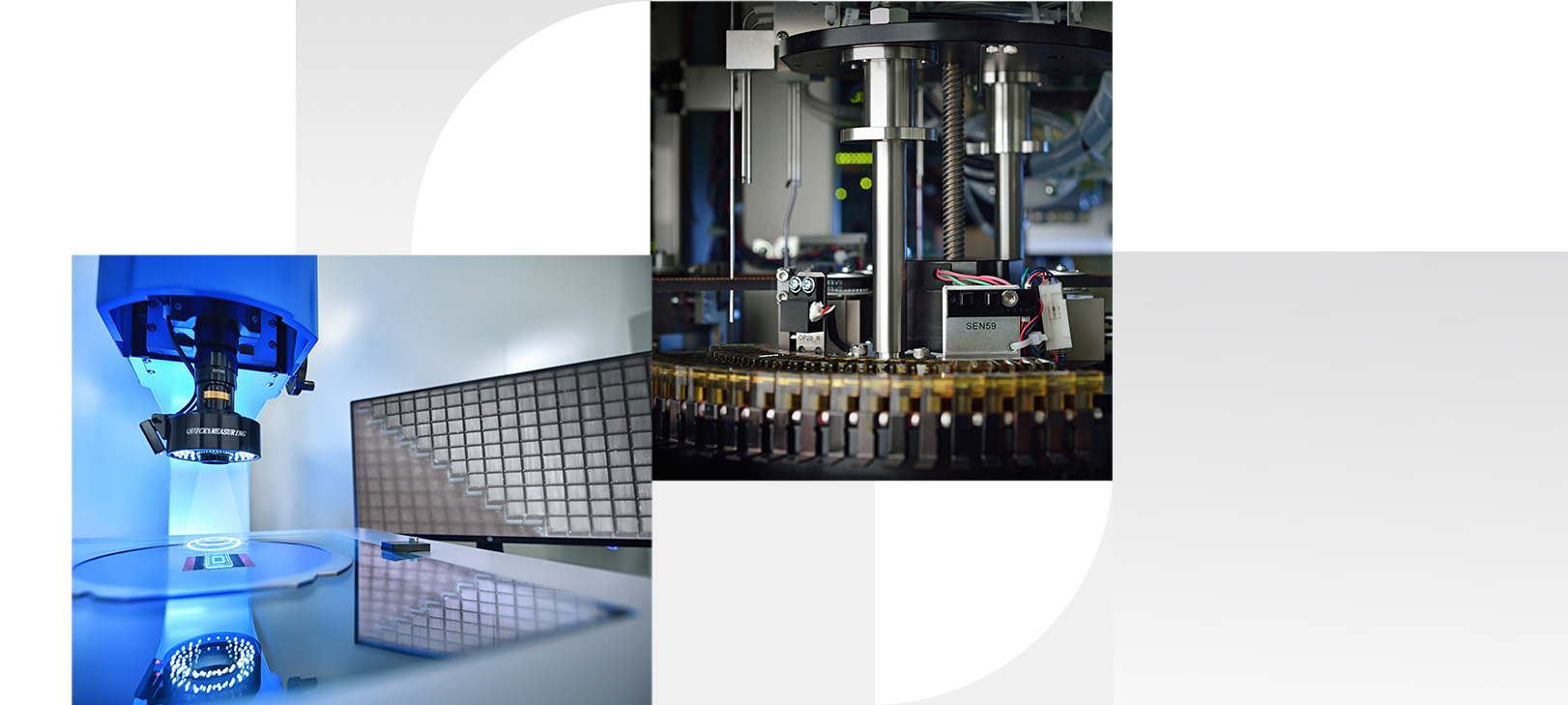
The design, development, verification, validation, processing and manufacturing of products need to be in line with the requirements set in the Reliability Guide to ensure reliable quality.
According to "Instrument, Equipment and Facilities Management Procedure", we conduct equipment life-cycle management from requirement collection, equipment procurement, design, validation, verification, maintenance, calibration and other aspects.
In the production process, the safety, quality, and other requirements in the "Internally Developed Product Standard" shall be implemented into the production process requirements and the requirements for equipment accuracy and reliability shall be implemented into the equipment management specifications. Regular maintenance of production equipment and calibration and verification of measuring equipment shall be carried out to ensure the safe and effective operation of equipment, effectively support the safe production and delivery inspection of products and archive the quality assurance of the whole manufacturing process ensures that each outgoing product meets the requirements of safety, performance and quality.

Advanced testing methods and strict execution
The birth of BeneVision N1 is the result of
{{times.toFixed(0)}}
times
of maximum energy charge and discharge
{{volt.toFixed(0)}}
volts
anti-static interference
{{bend.toFixed(0)}}
times
of cable bending
High-precision manufacturing technology covering the whole device and basic components
A Mindray Slide Maker is composed of
{{part.toFixed(0)}}+
components
The roughness of the blood cell blood valve is required to be
{{diameter.toFixed(2)}} μm
1/160 of the diameter of a red blood cell
Emergency Countermeasure
In order to timely respond to potential risks in product delivery, quality abnormality control, network security as well as other aspects, and ensure customer satisfaction and production continuity, the company has comprehensively identified risks, and accordingly the systematic countermeasures are formulated and implemented as follows.
Product delivery
In terms of material procurement: The Company has established the procurement mechanism for risk management and dual sourcing. It has used the diversified global supply network and strategic cooperation with key suppliers and prepared primary and alternative options for purchasing key materials to mitigate the risk of supply chain disruption and ensure the delivery of key materials.
In terms of production: Thehe Company has formulated emergency plans, including the Emergency Plan for Production Safety Accidents and Emergency Management of Electricity Use. It has also framed the Emergency Plan for Natural Disasters and Emergence Response Plan for COVID-19 to respond to climate change and COVID-19. Meanwhile, it has built and improved the layout of bases located at Guangming, Nanjing, Wuhan, Longhua and Dangshan. The Guangming base and Nanjing base are both put into use and others are still under construction. With these backup factories, the Company can prevent the potential risks in the production process and ensure the safety of employees and continuous production in the event of natural disasters, epidemics and abnormal accidents to guarantee the sustainable supply of products.
Quality abnormality control
For production, the Company has established the Nonconforming Product Control Procedure and the mechanism for suspension of delivery. It has established the corrective and preventive action management procedures to manage the possible anomalies in product quality promptly and effectively to ensure the safety and reliability of products. For clients, it has developed a global product recall mechanism to tackle the problems concerning product quality promptly so as to ensure product safety and minimize risks.
Cybersecurity
Management systems such as the Management System for Preventing Computer viruses, Management System for IT Application Data Backup and Specification for Information Security Aspects of Business Continuity Management. In this way, it can strictly protect the data and cyber security and prevent the occurrence of data leakage and other cyber security incidents so as to ensure the sustainability of cyber security and IT system.