Ultrasound Journal 36 - Detection of Hypoplastic Left Ventricular Syndrome in Second-Trimester Ultrasound Screening
2025-06-19
Special thanks to Esteban Lizárraga-Cepeda from Fetal Health Institute, Regional Maternal and Child Hospital, Monterrey, Nuevo León. Medical School, Vice-Rectory of Health Sciences, University of Monterrey, Gerardo Sepúlveda-González, Tayde Arroyo-Lemaroy, and Eduardo Nava-Guerrero from Fetal Health Institute, Regional Maternal and Child Hospital, Monterrey, Nuevo León. Tecnológico de Monterrey, School of Medicine and Health Sciencesfor showing the case.
Introduction
Congenital heart disease (CHD) is the leading cause of infant morbidity and mortality, with a worldwide prevalence of 8.2 per 1,000 live births. Arroyo et al. report a CHD rate of 5% in Mexico. Ultrasound screening and prenatal diagnosis can improve birth outcomes by enabling early intervention, allowing preparation for the birth of a neonate who will require specialized care and services, and facilitating early parental counseling. [1]
Hypoplastic left heart syndrome (HLHS) is a term that describes a spectrum of cardiac anomalies involving the underdevelopment of the left side of the heart. HLHS occurs in 1–2 per 10,000 live births. [2]
Prenatal screening includes risk detection for aneuploidy, preeclampsia, fetal growth restriction, and preterm delivery at 11-14 weeks, along with evaluation of fetal anatomy structures in the first trimester. A second-trimester assessment at 18-24 weeks includes screening for fetal abnormalities, malformations, placental evaluation, fetal growth curve, uterine artery Doppler screening for preeclampsia, and aneuploidy markers. [1]
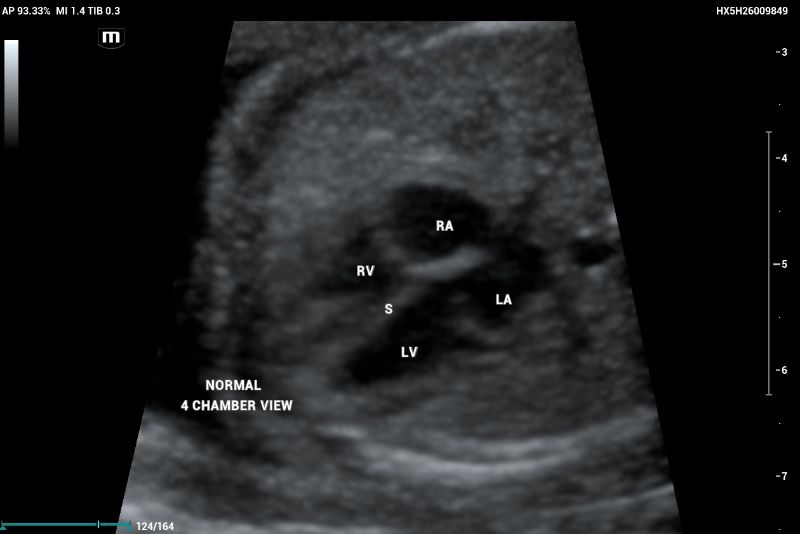
According to the ISUOG 2023 guidelines, second-trimester screening includes a standardized protocol for anatomical evaluation, incorporating fetal echocardiography. In a normal fetal echocardiogram, the ventricles should be symmetric and of equivalent size; any asymmetry is suggestive of disease (figure 1, and 2).
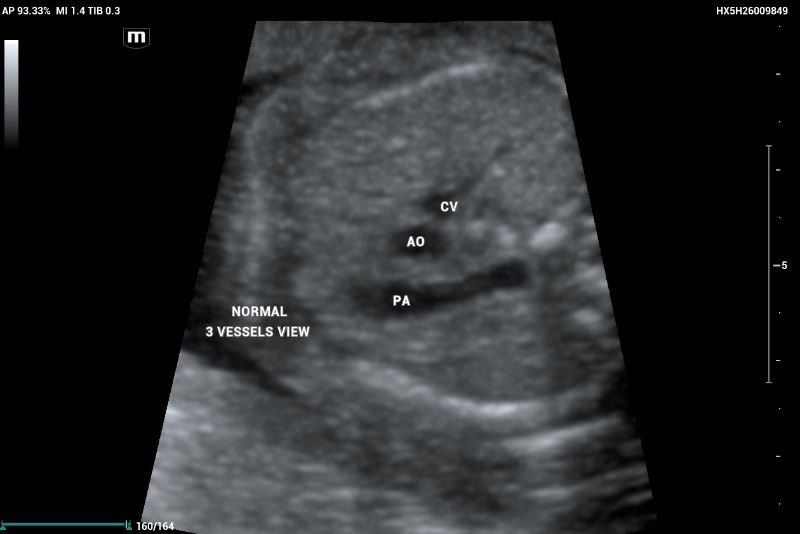
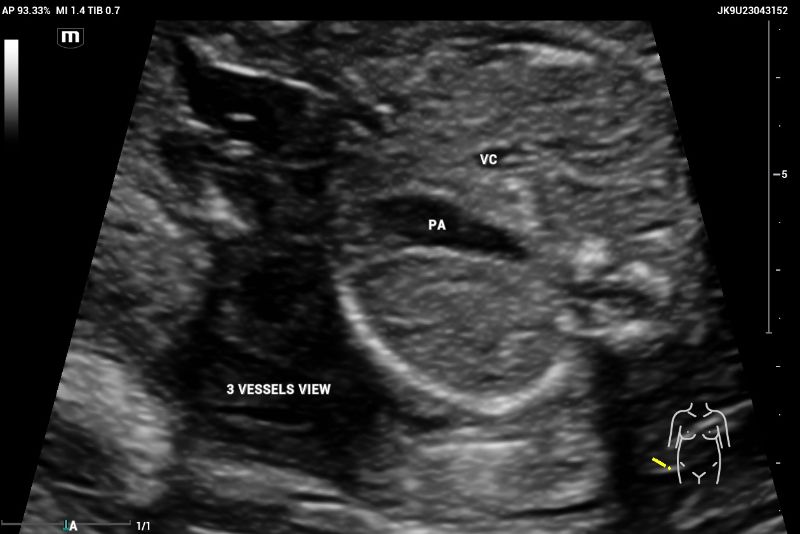
The three-vessel view (3VV) demonstrates the superior vena cava, aorta, and pulmonary artery, which should maintain a 1:1 size ratio (Figure 3).
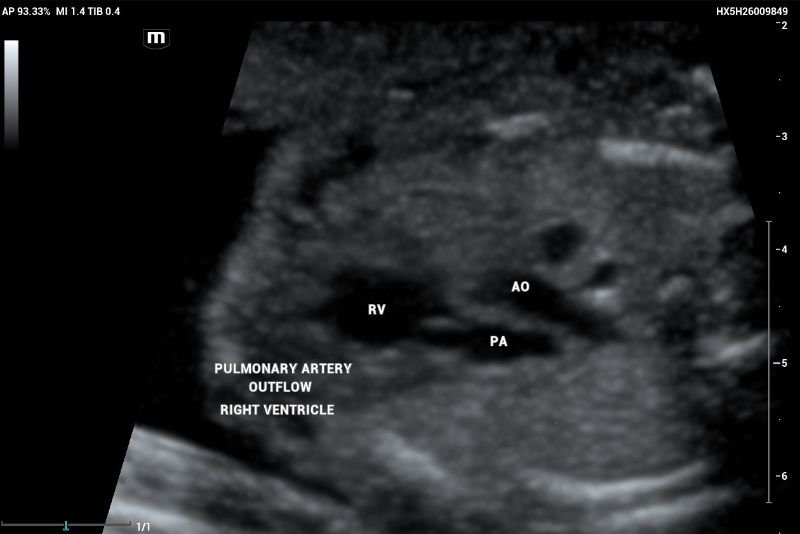
Outflow tract of aortic and pulmonary arteries and functional evaluation (Figure 4-6).
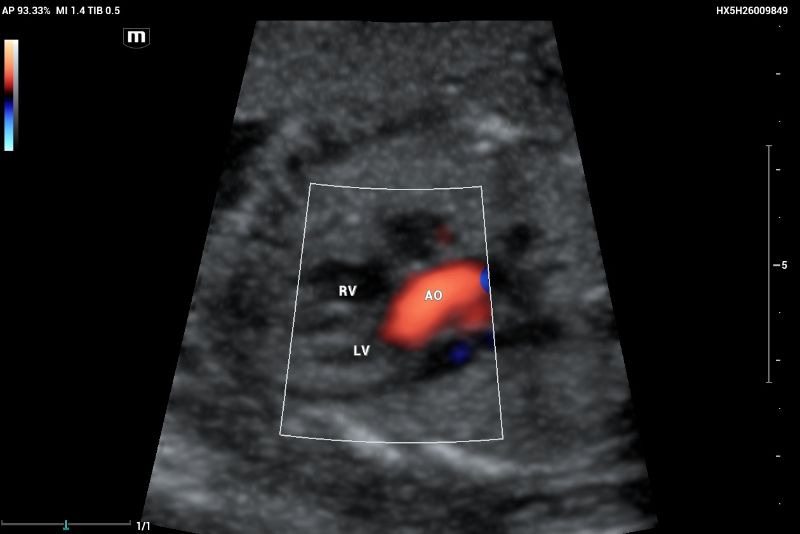
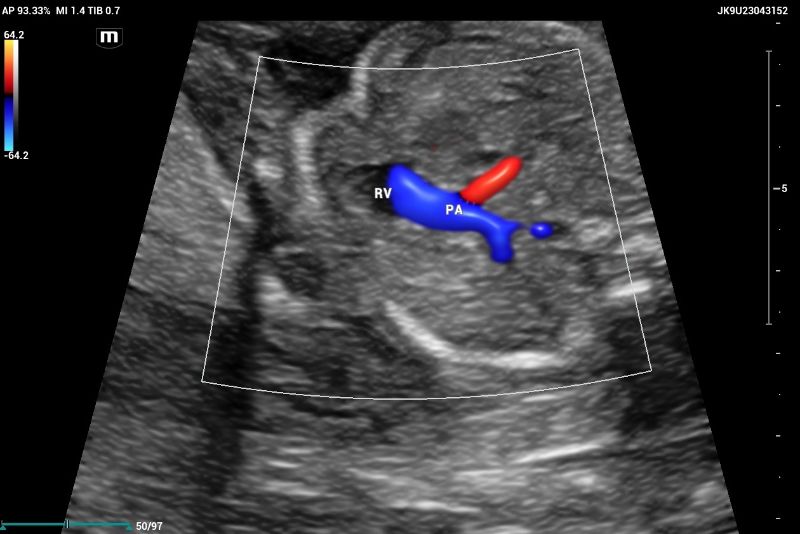
Clinical case
This is a 43-year-old female Hispanic woman, a nonsmoking, multiparous patient with one pregnancy loss, one vaginal delivery, and two cesarean deliveries. Her BMI is 31 kg/m², with a medical history of fibromyalgia (treated with pregabalin, which was suspended after the notification of pregnancy). She presented to the maternal-fetal medicine unit “ Fetal Health Institute” for a routine anatomy scan at 22 weeks and 2 days of gestation based on her last menstrual period.
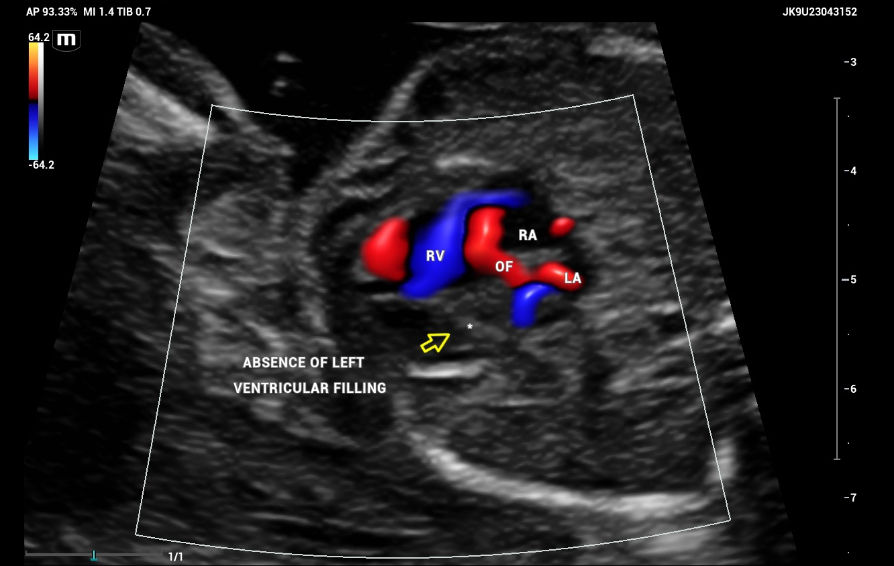
The ultrasound, performed on the Mindray Resona I9 system, showed an asymmetric four-chamber view. A single right ventricle was noted on the axial plane during the four-chamber view (4CV). The right ventricle appeared significantly larger than the left ventricle, with an absent left ventricular cavity. Only the main pulmonary artery (PA) and the vena cava could be identified on the three-vessel view (3VV)( Figures 7 and 8).
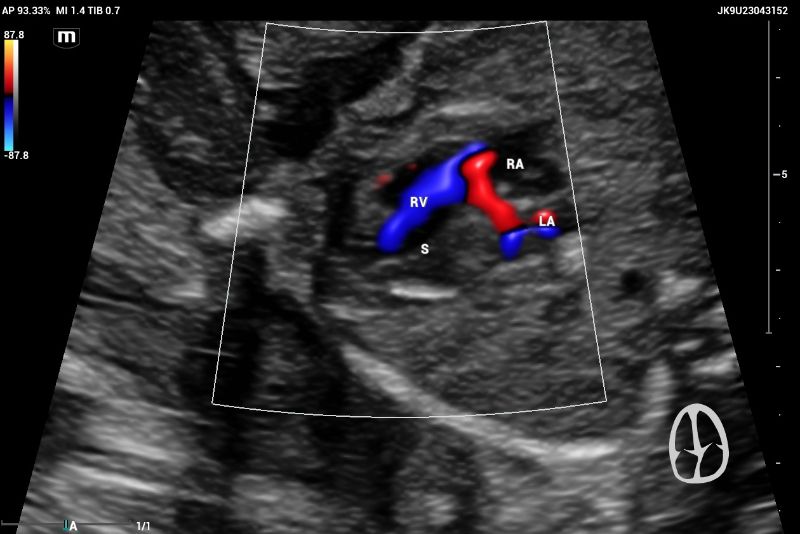
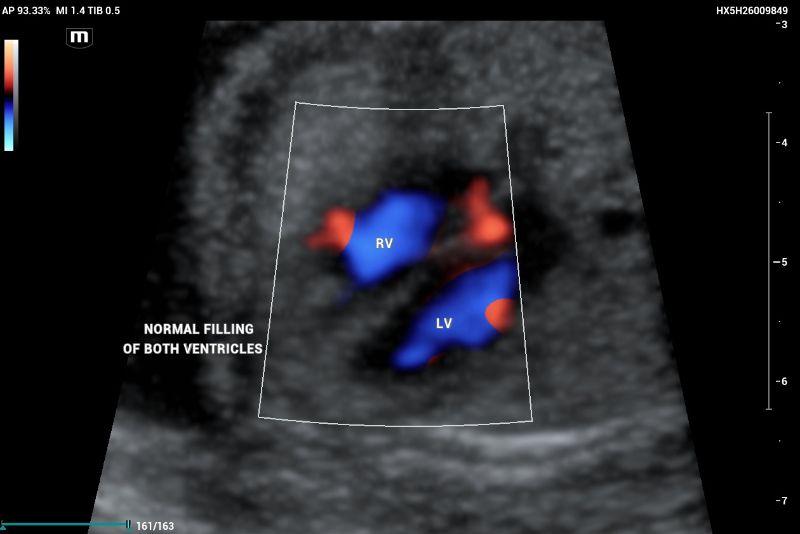
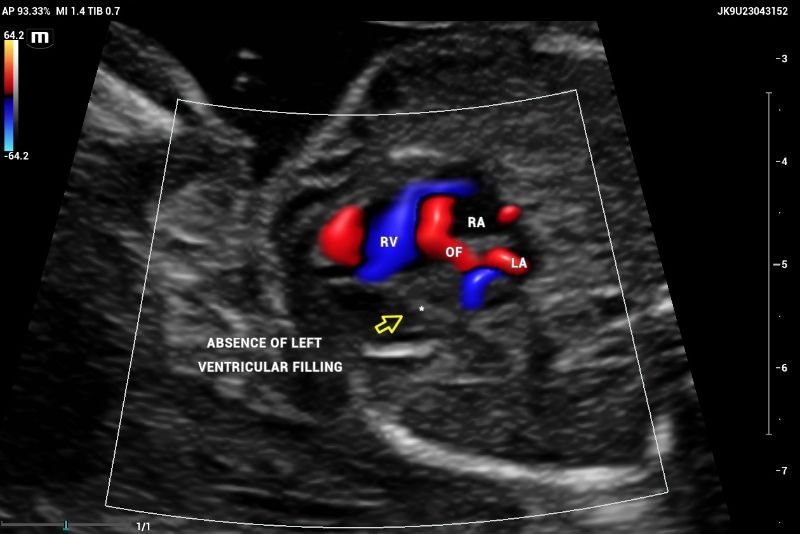
The emergence of the aorta was difficult to visualize, and hypoplasia or aortic atresia was suspected during the exam. Color and power Doppler were used to visualize systolic and diastolic flow to obtain ventricular filling images. Absent color Doppler could be visualized by entering the LV through the mitral valve(Figure 9), in contrast to a normal echocardiography showing normal filling and size of symmetric ventricles.
Discussion
Cardiovascular anomalies are the most common major congenital anomalies. At birth, the incidence of cardiovascular anomalies is 6.5 times higher than chromosomal anomalies and up to four times more common than neural tube defects. Congenital cardiac anomalies account for up to 20% of neonatal deaths and 50% of infant deaths and are four to five times more prevalent in stillbirths than in live-born infants. [3]
Hypoplastic left heart syndrome (HLHS) accounts for up to 9% of CHDs and results in inadequate systemic circulation after birth. The spectrum of cardiac malformations is characterized by significant underdevelopment of the components of the left heart and the aorta, left ventricular cavity, and mass, including mitral atresia or stenosis, aortic stenosis or atresia, and hypoplasia of the left ventricle. The right ventricle maintains fetal circulation as a single functional ventricle and usually forms the apex of the heart in these cases. HLHS does not cause any adverse effect in utero because a single healthy ventricle can compensate for a hypoplastic contralateral ventricle. After birth, this syndrome is ductal-dependent [2]. In cases with a severe absence of tricuspid regurgitation, the fetus could be delivered at term. [3-5]
HLHS can be detected on prenatal sonography between 18 and 22 weeks of gestation with a 4-chamber view of the fetal heart. Early prenatal detection in the second trimester at 18-22 weeks has been shown to improve outcomes compared with neonates without a prenatal diagnosis. Second-trimester ultrasonography had a sensitivity of 31 to 61.9% for isolated left heart syndrome detection. Prenatal diagnosis can demonstrate a dysfunctional left ventricle and critical aortic stenosis and/or severe coarctation of the aorta. A protocolized study of echocardiography shows images of obstructed outflow and dysfunctional contractility of the left ventricle wall. Fetuses with HLHS can develop endocardial fibroelastosis, increasing cardiac wall echogenicity, and the left ventricle diminishes in size relative to the normally growing right ventricle. In utero aortic valve dilation has been proposed for cases with critical aortic stenosis. [3-6]
Prenatal diagnosis and multidisciplinary management are necessary to improve outcomes, after birth, meaning closure of the PDA will result in a decrease in pulmonary vascular resistance, which could progress to hemodynamic shock. Ductal patency is necessary with the administration of prostaglandin E1. Almost 23% of all cardiac deaths that occur in the first week are associated with HLHS and have a 100% mortality without treatment because, after birth, the left ventricle is unable to support systemic circulation. Neonatal surgery is the most common treatment. There are different palliative surgical procedures, which include aortic valvuloplasty, bidirectional cavo-pulmonary shunt, modified Fontan procedure, and modified Norwood procedure, increasing the survival rate. The therapeutic approach is initially staged surgical palliation, and some cases require cardiac transplantation. Fetal therapy with aortic valvuloplasty may be considered in fetuses showing characteristics of a dilated left ventricle with poorly contracting, foramen ovale showing left-to-right or bidirectional shunting, and retrograde aortic arch flow. [2-6]
Conclusion
The use of ultrasound to detect fetal malformations at the second trimester screening may lead to improved outcomes in congenital heart disease cases. Early detection and timely referral are necessary to manage HLHS. Prenatal diagnosis is important for pregnancy counseling and for planning the delivery location and timing. A prompt referral improves maternal-fetal outcomes due to the severity of this condition and the specialized surgical treatment that is required at the tertiary care level. In cases with critical aortic stenosis and tricuspid regurgitation, fetal surgery is an option in specialized centers with a multidisciplinary team including fetal surgeons, neonatologists, pediatric cardiologists and surgeons, and an intensive care unit with the specific infrastructure for prenatal and neonatal care.
References
[1]. Carvalho JS, Axt-Fliedner R, Chaoui R, Copel JA, Cuneo BF, Goff D, Gordin Kopylov L, Hecher K, Lee W, Moon-Grady AJ, Mousa HA, Munoz H, Paladini D, Prefumo F, Quarello E, Rychik J, Tutschek B, Wiechec M, Yagel S. ISUOG Practice Guidelines (updated): fetal cardiac screening. Ultrasound Obstet Gynecol. 2023 Jun;61(6):788-803.
[2]. Hildebrand E. First-Trimester Diagnosis of Hypoplastic Left Heart Syndrome: A Case Report. Journal of Diagnostic Medical Sonography. 2021;37(2):185-192.
[3]. Sadineni RT, Kumar BS, Chander NB, Boppana DM. Prenatal Sonographic Diagnosis of Hypoplastic Left Heart Syndrome. Int J Appl Basic Med Res. 2017 Jul-Sep;7(3):213-215.
[4]. Tulzer A, Huhta JC, Hochpoechler J, Holzer K, Karas T, Kielmayer D, Tulzer G. Hypoplastic Left Heart Syndrome: Is There a Role for Fetal Therapy? Front Pediatr. 2022 Jul 8; 10:944813.
[5]. Najib B, Quibel T, Tessier A, Mortreux J, Bouvagnet P, Cohen C, Vialard F, Dard R. Prenatal diagnosis of recurrent hypoplastic left heart syndrome associated with MYH6 variants: a case report. BMC Cardiovasc Disord. 2023 Mar 8;23(1):116.
[6]. Mustafa HJ, Aghajani F, Jawwad M, Shah N, Abuhamad A, Khalil A. Fetal cardiac intervention in hypoplastic left heart syndrome with intact or restrictive atrial septum, systematic review, and meta-analysis. Prenat Diagn. 2023 Aug 19.