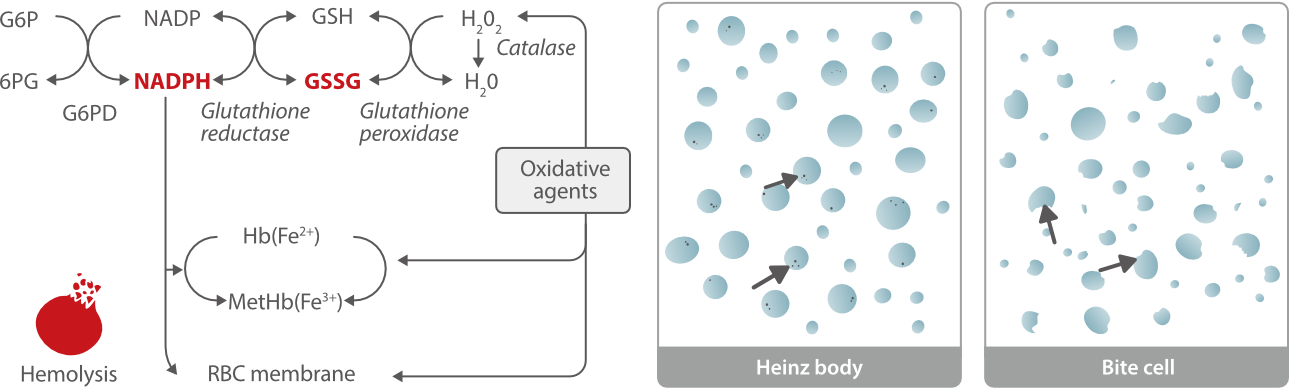
Glucose-6-phosphate dehydrogenase (G6PD) is the rate-limiting enzyme of the pentose phosphate pathway. In this metabolic pathway, G6PD supplies reducing energy to red blood cells (RBCs) through NADPH and glutathione (GSSG), which can protect RBCs from oxidative stress. Decreased G6PD activity can trigger hemoglobin denaturation (Heinz body and bite cell in blood smears) and membrane damage (hemolysis) in RBCs. [1, 2]
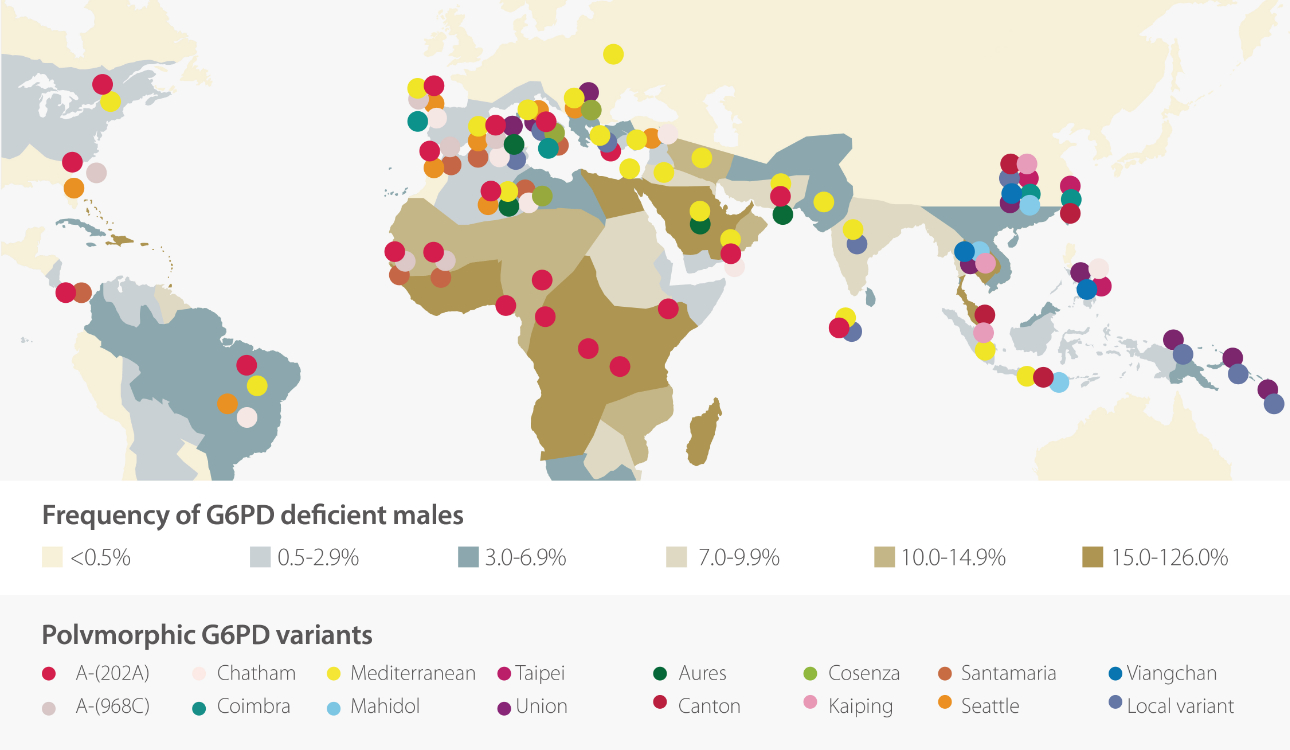
G6PD deficiency, an X-linked recessive disorder, is the major cause of decreased G6PD activity. G6PD deficiency can be caused by more than 180 gene mutations. [3] It is the most commonly observed enzyme deficiency in the world. More than 400 million people are experiencing G6PD deficiency. [4] It has been considered highly related to malaria and there is considerable geographical overlap between the prevalence of G6PD deficiency and that of P. vivax. [5]
For the diagnosis of G6PD deficiency, medical practitioners shall refer to the symptoms, signs, and family histories of the patient, as well as his/her laboratory examination results, such as liver function assay, LDH, blood count, Combs tests, gene analysis, and G6PD activity testing. The gene mutations do not change in a single patient, especially a female patient, but the G6PD activity changes according to the patient’s status. This makes G6PD activity the most essential laboratory testing, not only for the diagnosis of G6PD deficiency but also for screening and assessment.
Under various conditions, individuals may take the G6PD activity testing, especially for those with favism, family history of G6PD deficiency, neonatal hyperbilirubinemia, acute or chronic hemolysis, hemoglobinuria, thalassaemic disorders, and hemolysis with unknown causes, as well as before the administration of certain medications (e.g. primaquine, sulfamethoxazole, quinolones, dapsone, and nitrofurantoin). [4] [7]
Based on the principle of the assay, G6PD activity testing can be classified as qualitative assay and quantitative assay.
Qualitative assays, such as the fluorescent spot test (FST) and dye reduction methods, have been developed for a variety of conditions and purposes. Qualitative assays are simple and less expensive, so these assays are applied for screening newborns in some nations. This is especially important when G6PD deficiency is potentially suggested by family history, ethnic or geographic origin, or the timing of the appearance of neonatal jaundice. [7, 8]
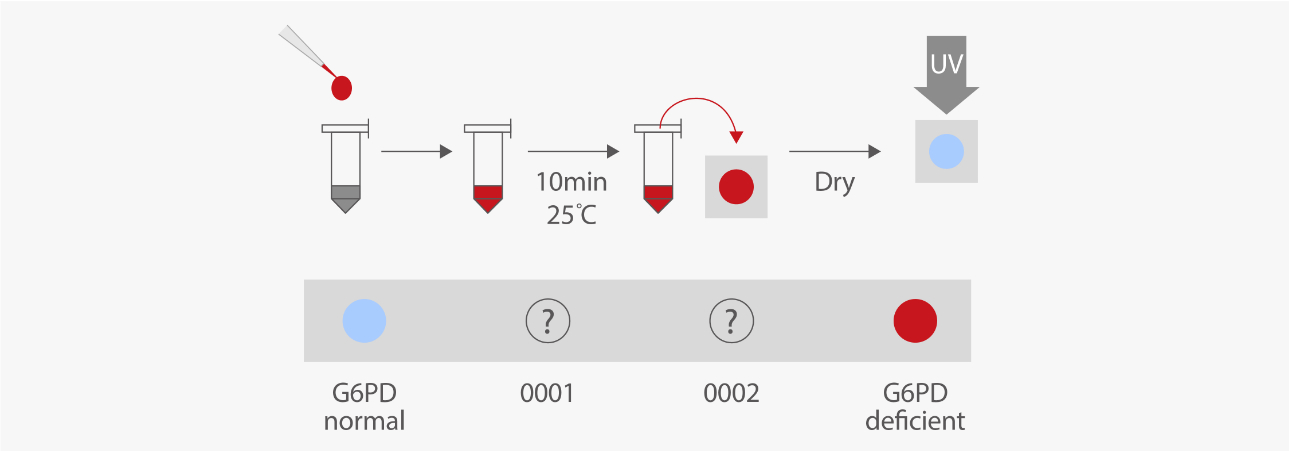
However, qualitative assays cannot provide sufficient information for females. Unlike G6PD in males, G6PD in females is more complicated because of the X-linked recessive hereditary pattern and the random inactivation mechanism of X-chromosomes. The G6PD activity in female carriers can be deficient, intermediate (partially deficient), or normal, and these activities may change over time even in the same female carrier. [9]
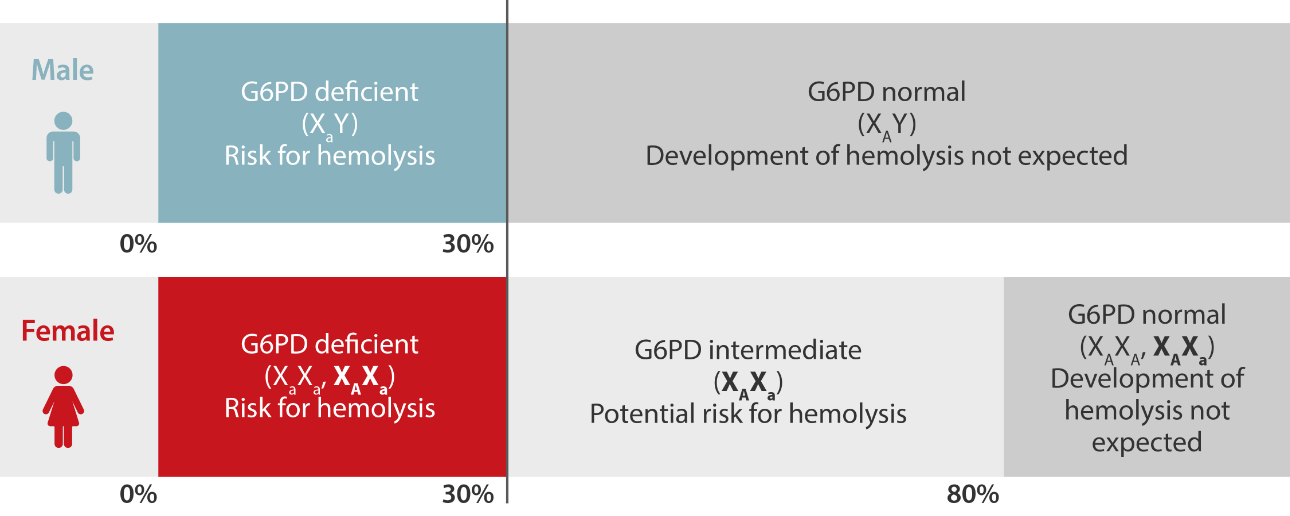
Therefore, the guideline from the British Society for Hematology suggested offering quantitative assays to female patients directly, since these patients may be heterozygous and misclassified by screening tests. Quantitative assays are also recommended for those with abnormal or equivocal qualitative assay results so as to verify the diagnosis. [10]
The mechanism of the quantitative assay is mainly based on light absorption. Nicotinamide adenine dinucleotide phosphate (NADP) is reduced to NADPH by G6PD with the presence of its specific substrate, glucose-6-phosphate. The enzyme activity can be determined by measuring the changes in absorbance rate at 340 nm due to the reduction of NADP.
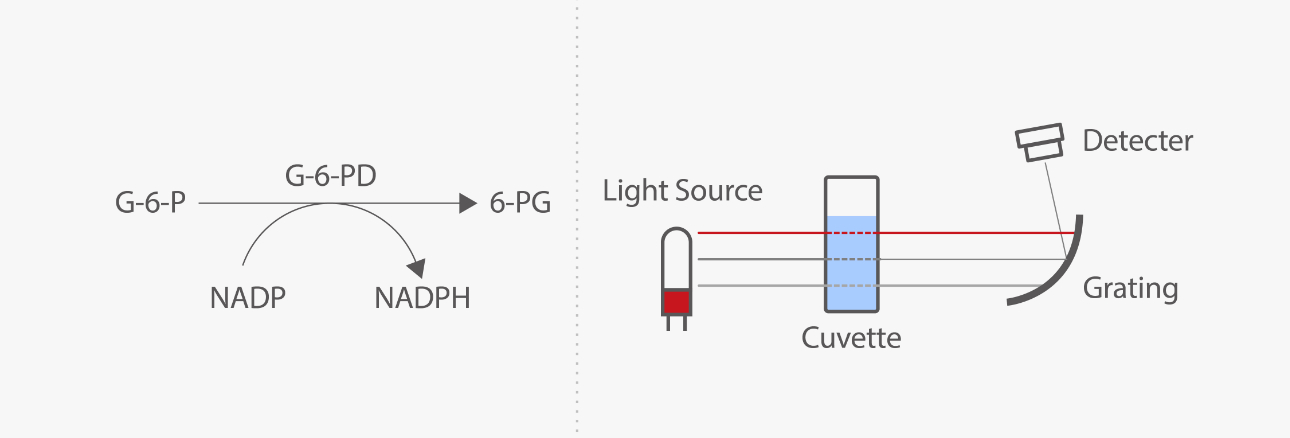
G6PD activity quantitative assay shall be conducted under strict prerequisites, including blood collection, sample storage, and reaction temperature. Here's an example. Accurate cuvette temperature control is very important for the quantitative assay because the G6PD activity testing is highly temperature-dependent. Laboratories need to measure the temperature inside the reaction cuvette or use an automated analyzer, no matter whether the assay is running at 30 °C or 37 °C. [10]
According to a systematic review, we can see that the variations in G6PD activity are significantly different among studies due to different ethnic groups and populations.
These variations are also observed even when using the same commercialized quantitative assay kit. [11]
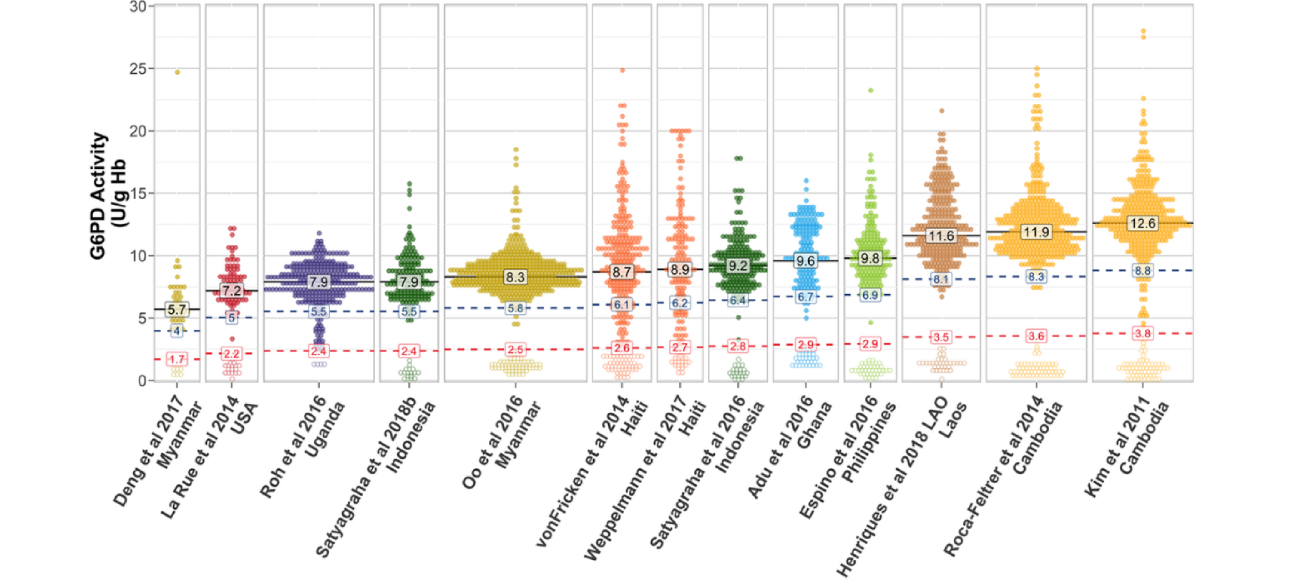
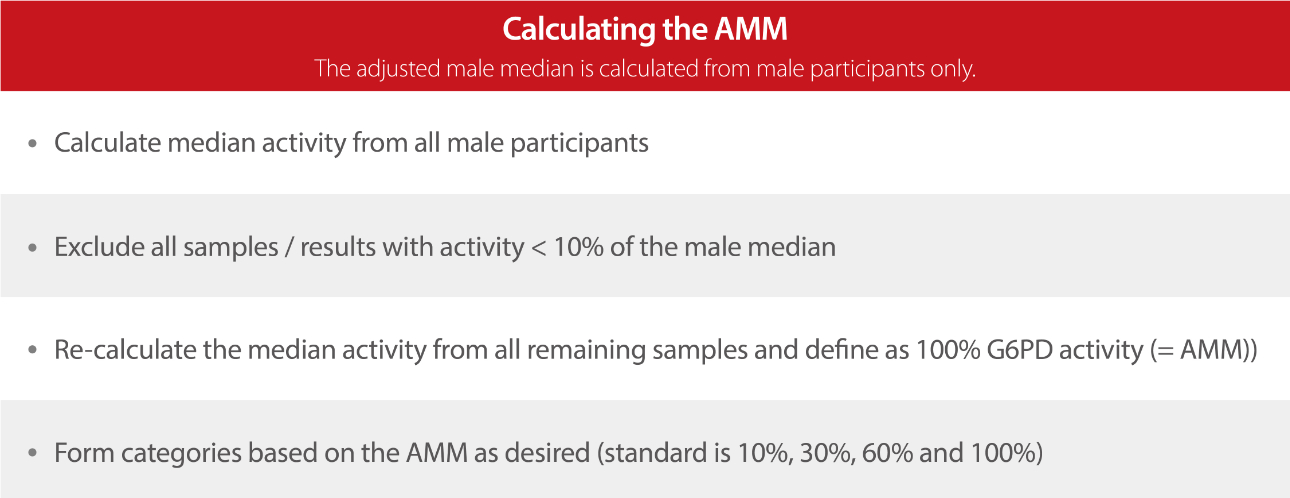
Therefore, it is important to determine the reference interval of G6PD activity in each laboratory by either using an in-house procedure or a commercialized kit. [10] The G6PD reference interval can be determined by defining assay-specific and population-specific absolute values that represent 100% G6PD activity. The following procedure is used to calculate the adjusted male mean (AMM). [12]
In conclusion, G6PD deficiency is currently a common disorder in the world and extra attention should be paid to the areas and regions with high prevalence. Individuals with G6PD deficiency can benefit from G6PD activity testing, especially the quantitative assay, for the diagnosis of G6PD deficiency, instruction of medications, and prevention of hemolysis.
Reference
[1] Ford, J. (2013). "Red blood cell morphology". International Journal of Laboratory Hematology. 35 (3): 351–357. doi:10.1111/ijlh.12082.
[2] "Glucose-6-phosphate dehydrogenase deficiency: MedlinePlus Genetics". medlineplus.gov. Retrieved 2022-03-21.
[3] Gómez-Manzo, S. et al. Glucose-6-Phosphate Dehydrogenase: Update and Analysis of New Mutations around the World. International Journal of Molecular Sciences 17, 2069 (2016).
[4] Cappellini, M. D., Fiorelli, G. Glucose-6-phosphate dehydrogenase deficiency. Lancet 2008; 371: 64–74.
[5] Howes RE, et al. G6PD deficiency prevalence and estimates of affected populations in malaria endemic
countries: a geostatistical model-based map. PLoS Med. (2012) 9: e1001339. 10.1371/journal.pmed.1001339
[6] Luzzatto L, Notaro R: Malaria Protecting against bad air. Science 2001, 293:442–443.
[7] Jennifer E. Frank Diagnosis and Management of G6PD Deficiency. Am Fam Physician. 2005;72:1277-82.
[8] Baird, J.K., et al. Glucose-6-Phosphate Dehydrogenase Deficiency and Primaquine Hemolytic Toxicity. Encyclopedia of Malaria. 2014. https://doi.org/10.1007/978-1-4614-8757-9_102-1
[9] Testing for G6PD deficiency for safe use of primaquine in radical cure of P. vivax and P. ovale malaria. WHO. https://apps.who.int/iris/rest/bitstreams/1061439/retrieve
[10] Roper, D. et al. Laboratory diagnosis of G6PD deficiency. A British Society for Hematology Guideline. Br J Haematol 189, 24–38 (2020).
[11] Pfeffer, D. A. et al. Quantification of glucose-6-phosphate dehydrogenase activity by spectrophotometry: A systematic review and meta-analysis. PLoS Med 17, e1003084 (2020).
[12] Ley, B. et al. Methods for the field evaluation of quantitative G6PD diagnostics: a review. Malar J 16, 361 (2017).